
Taxonomic description of species
The shell is rounded or oval. The sinus is rather small, hardly goes behind 1/3 of the shell length. The surface of the shell is covered with pronounced ribs. They are usually more than 20-22 in number (except for the marginal ones hardly noticeable)
Intraspecific forms. Hypanis angusticostata is represented in the Caspian Sea by two subspecies different from those inhabiting the Black Sea:
Hypanis angusticostata polymorpha Logv. et Star., Hypanis angusticostata acuticostata Logv. et Star.
Related forms. Hypanis is widely spread in water bodies of the Azov and Black Sea basins:
Hypanis colorata Eichwald, Hypanis caspica Sc. et Star., Hypanis pontica
(Eichwald).
Distribution of species within the Caspian Sea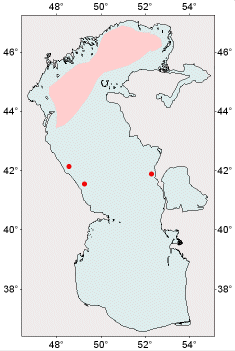
The main habitat of Hypanis angusticostata is the Northern Caspian. It is not abundant in the Middle Caspian and occurs at depths not more than 50 m. The species was not seen in the southern part of the sea including Iranian waters. Wide spreading of the mollusk in the Northern Caspian begins during the period of its most active breeding in June. Since August, the areas occupied by the species have been reduced.
The highest densities of the mollusk were recorded in the central part of the Northern Caspian during the whole vegetative period.
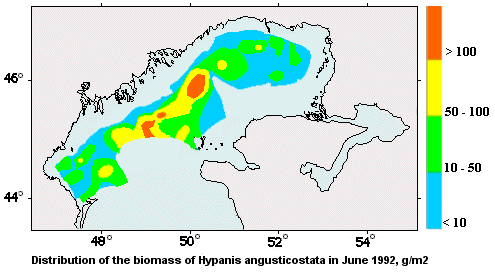
Status as per International Red Data Book. Not defined
Status as per National Red Data Books. Not defined
First record for the Caspian Sea. Pallas, 1771
Redescription of species. Eichwald, 1838; Grimm, 1877; B.M. Logvinenko and Ya.I. Starobogatov, 1967.
General characteristics of species
Ecological-taxonomic group. Macrozoobenthos
Origin. Autochthonous Caspian species
World distribution. The Caspian endemic. At present it occurs in the Caspian, Azov, and
Black Seas (Mordukhay-Boltovskoy, 1978).
Habitat. Hypanis angusticostata inhabits the surface layer of the ground. The mollusk half or completely buries itself in the ground leaving exposed its
siphons (Romanova, 1963). It lives in the grounds covered with silt, hard sand and shell rock, its abundance is dependent on the concentration of detritus in the soil. The mollusk occur in silt-covered grounds (Sayenkova, 1951).
The high biomass of the mollusk is constantly recorded in the areas of the Northern Caspian in front of the Volga delta, which have large amounts of organic matter in suspension (2-5 mg/l) and its easily hydrolyzed fractions (1-3 mg/l).
Migrations. Information is not available
Relation to abiotic environmental factors
Relation to salinity. Brackish water euryhaline species
It occurs in the Caspian Sea at a salinity of 1-14o/oo. The optimum values
are within
6-10o/oo, the sublethal salinity is 2-4o/oo and the lethal one is 0 and
15o/oo (Karpevich, 1946)
Relation to temperature. Eurythermic species
The first larvae appear at a water temperature 8-110C, the maximum number is
recorded at
20-240C (Galperina, 1976).
Vertical distribution. Hypanis occurs in the Northern Caspian practically
at all depths. It was not seen only in the deepwater zone and in some shallow estuarine areas
close to the delta. Maximum densities of the mollusk were recorded at a depth 3-6
m (up to 7, 500
sp./m2 or 1.3 kg/m2).
In the Middle Caspian, the species inhabits adepths down to 50 m from the
surface.
Relation to oxygen conditions. For the mass development of Hypanis
angusticostata, the oxygen content needs to be more than 1.0-1.3 cm3/l (Maximova, 1953).
Relation to fluctuations of the sea level. In 1977, at a low seawater level (29.0 m abs.), the mean biomass of the mollusk in the Northern Caspian amounted to 6.4
g/m2. During the period of a rise in seawater level (1979-1995) to -26.54 m abs., the biomass increased to 9.1
g/m2, during 1996-2000 (the period of the next drop in seawater level to
-27.1 m abs.), the biomass decreased to 4.9
g/m2 (data by Ossadchikh and Malinovskaya).
Feeding
Feeding type. Heterotrophic
Feeding behavior. Filter feeder
Food spectrum. Stenophagous species
The source of food is suspended nutritive substances in water and on the surface of sediment. Various species of planktonic algae were found in the intestine of
Hypanis from the south-western and central parts of the Northern Caspian. Beside
Exuviaella cordata and Actinocyclus ehrenbergii, there also occurred small species of green, blue-green and diatom algae and brown organic-mineral aggregations.
Intestinal content of Hypanis angusticostata
in the Northern Caspian (Yablonskaya, 1971)
| Date |
June, 1958 |
August, 1959 |
| Frequency of occurrence |
% |
% |
| Rhizosolenia calcar-avis |
10 |
20.8 |
| Actinocyclus ehrenbergii |
100 |
87.5 |
| Sceletonema costatum |
30 |
- |
| Sceletonema subsalsum |
10 |
- |
| Fragillaria sp. |
30 |
- |
| Thalassiosira variabilis |
50 |
50.0 |
| Melosira |
50 |
- |
| Navicula |
20 |
- |
| Diatoma elongatum |
20 |
- |
| Exuviaella cordata |
- |
50.0 |
| Pidiastrum sp. |
70 |
- |
| Scenedesmus quadricauda |
90 |
45.8 |
| Binuclearia |
100 |
100,0 |
| Oocystis |
30 |
- |
| Conferva |
40 |
- |
| Spores |
10 |
- |
| Coeloshaerium |
10 |
- |
| Aphanizomenon issatschenkoi |
10 |
29.2 |
| Microcystis pulverea |
20 |
83.3 |
| Merismopedia punctata |
80 |
87.5 |
| Gloocapsa |
10 |
45.8 |
| Gomphosphaeria |
- |
12.5 |
| Lyngbia |
- |
22.7 |
| Particles of macrophytes |
40 |
- |
| Dense amorphous brown clusters |
90 |
25.0 |
| Light fine-grained flakes |
- |
45.8 |
| Mineral suspension |
- |
4.2 |
| Number of specimens |
10 |
46 |
| Size of mollusks, mm |
9-27 |
7-19 |
The major food of the mollusk in the Middle Caspian is detritus, mostly planktonogenic with a plenty of remnants (small spines) of
Rhizosolenia. Selective ability in this species is less pronounced than in other filter feeders.
Intestinal content of Hypanis angusticostata
in the Middle Caspian in summer, 1962 (Yablonskaya, 1971)
| Components |
% |
| Fine-grained detritus |
33.1 |
| Small algae (mainly Exuviaella cordata) |
5.9 |
| Large algae (Rhizosolenia, Cosuinodiscus) |
7.2 |
| Organic-mineral dense brown aggregations |
33.0 |
| Mineral suspension |
20.8 |
| Number of Exuviaella cells per 1 mg of intestinal content |
685 |
| Number of Rhizosolenia spines per 1 mg of the intestinal content |
5423 |
| Number of Hypanis angusticostata specimens |
311 |
| Number of specimens with empty intestines |
0 |
| Length of mollusks, mm |
12-24 |
Supply of food. Phytoplankton, organic detritus, mineral suspension.
Quantitative characteristics of feeding. The filling rate in the anterior part of the digestive tract is very high in case of suspended food and very low if food objects lie on the ground surface.
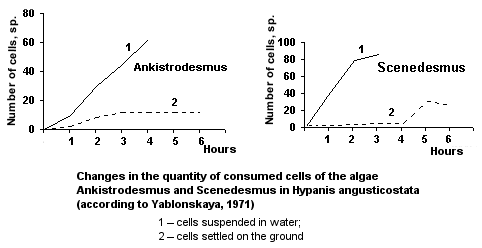
When the density of algae is equal to 153, 000 cells/ml, the rate of filtration constitutes 10.43 ml/hr, at the density 50, 000 cells/ml, the rate is 25.83 ml/hr (Sanina, 1975).
Relationship between filtration rate (F1, ml/sp. per hour) and body dry weight (W, mg)
in Hypanis angusticostata, Northern Caspian, at various concentrations of food
(F=anb)
| Algae density |
Relationship parameters |
| thou. cells per 1 ml |
mg of dry matter per 1 l |
cal/l |
� |
b |
F1 |
| 153 |
18.36 |
73.44 |
1.401 |
0.880 |
10.43 |
| 85 |
10.20 |
40.80 |
2.599 |
0.729 |
13.73 |
| 50 |
6.00 |
24.00 |
5.042 |
0.716 |
25.83 |
Note: F1 � filtration rate (ml/hr calculated for Wdry=9.8 mg
Concentrations of suspended organic matter necessary to meet nutritional requirements of
Hypanis angusticostata are estimated to be 8.52 cal/l at a maximum rate of filtration and 21.09 cal/l at a minimum one.
Calculation of feeding ration for Hypanis angusticostata (similar-sized mollusks), Northern Caspian
| Parameters |
|
| Body weight, mg |
|
| dry |
9.8 |
| fresh |
84.92 |
| Dry matter, % of fresh body weight |
11.54 |
| Oxygen consumption, mg O2/sp. per hour |
0.022 |
| Energy consumption (metabolism), cal/sp. per hour |
0.077 |
| Energy consumption (metabolism and growth), |
0.154 |
| Ration at A=0.7 cal/sp. per hour |
0.220 |
Calculated concentration of suspended organic matter necessary to meet nutritional requirements of
Hypanis angusticostata in the Northern Caspian (Sanina, 1975)
| Parameters |
Value |
| Ration, cal/sp. per hour |
0.220 |
| Filtration rate, ml/sp. per hour |
|
| maximum |
25.83 |
| minimum |
10.43 |
| Food concentration needed, cal/l |
8.52/21.09 |
| �org, mg/l |
0.91/2.25 |
* Note: Numerator values correspond to the maximum, denominator � to the minimum rate of filtrati.
Reproduction
Reproduction type. Gamogenesis
Hypanis angusticostata is a dioecious mollusk. The fertilization of eggs occurs in water.
Reproduction areas. There are no specific areas of reproduction in the Caspian Sea.
Terms of reproduction. Reproduction begins in late April when the water temperature increases
up to
11.5
0C, continues through all the summer at water temperature up to
24
0C, and ends by September when the water temperature decreases to
19.2
0C (Galperina, 1972).
Spawning is intermittent. After spawning, recovery of gonads occurs.
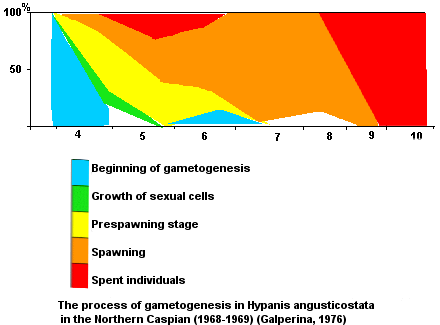 Fecundity.
Fecundity. The fecundity of the species may reach several hundred thousand eggs. Because of the great extension and continuity of
oogenesis, the exact number of eggs was not determined.
Limiting factors.
The factors affecting the reproduction of
Hypanis angusticostata is water temperature, salinity,
conditions/ food availability.
Life history and development
Life-history stages. Development of eggs occurs similar to most marine bivalve mollusks: cleaving egg develops into the first larval stage � trochophore that turns into a veliger larva, which has a bivalve shell and a velum.
The pelagic period of a larva lasts about a month in spring and not less than two weeks in summer. After that period, larvae settle on the bottom and turn into young mollusks (Galperina, 1976; Maximova, 1953).
Relation to environmental factors. Reproduction season is confined mainly to warmer seasons - spring and summer, at a water temperature
20-250C. The spawning interval (late autumn, winter and early spring) occurs at rather low water temperature
(5-100C).
Larvae are found in waters of wide salinity range, from 4 to 12o/oo (Galperina, 1976).
Age of maturity. Hypanis angusticostata usually begins
reproduction in the second year of its life when its shell length reaches 7-9 mm.
Thermal conditions of development. The first larvae appear in the Northern Caspian at a temperature
8-100C (Galperina, 1976)
Quantitative characteristics of growth. N/A
Structural and functional population characteristics
Sex ratio. N/A
Age-size structure. The reproduction of the Caspian Hypanis angusticostata begins in early
spring, which is confirmed by large quantities of juveniles less than 3 mm in length appearing in April
Size composition of Hypanis angusticostata in the Northern Caspian
| Size, mm |
<3 |
3.1-5 |
5.1-7 |
7.1-9 |
9.1-11 |
11.1-13 |
13.1-15 |
15.1-17 |
17.1-19 |
19.1-21 |
21.1-23 |
23.1-25 |
25.1-27 |
| April |
1587 |
374 |
69 |
|
23 |
20 |
22 |
24 |
10 |
17 |
2 |
- |
- |
| May |
179 |
108 |
35 |
79 |
60 |
55 |
44 |
31 |
14 |
11 |
4 |
4 |
- |
| June |
98 |
85 |
188 |
73 |
75 |
54 |
64 |
32 |
12 |
19 |
5 |
1 |
- |
| July |
461 |
13 |
52 |
42 |
45 |
32 |
35 |
64 |
20 |
30 |
10 |
7 |
1 |
| August |
232 |
81 |
15 |
4 |
6 |
16 |
26 |
13 |
8 |
9 |
4 |
- |
- |
| September |
424 |
95 |
37 |
31 |
16 |
23 |
19 |
12 |
21 |
11 |
- |
3 |
- |
| October |
928 |
191 |
55 |
31 |
24 |
19 |
5 |
11 |
7 |
3 |
- |
- |
- |
(unpublished data by Ossadchikh, 1974)
In May-June its reproduction activity decreases while young mollusks that appeared earlier grow rapidly. The mean size of individuals at that period reaches its maximum.
In the second half of the year, beginning June, the number of individuals less than 3 mm in length increases again considerably while the mean length decreases.
Quantitative characteristics. In the 20th century the biomass of Hypanis angusticostata in the Northen Caspian varied within the following
range:
| 1930s |
1940s |
1950s |
1960s |
1970s |
1980s |
1990s |
| 2.2 g/m2 |
3.3g/m2 |
6.7 g/m2 |
4.1g/m2 |
5.6 g/m2 |
9.2 g/m2 |
8.1 g/m2 |
Population trends. Since 2000 the population number of Hypanis angusticostata has declined.
Interspecific relations
Being a filter feeder, Hypanis angusticostata is a food competitor to
the other mollusks of the Caspian Sea
(Didacna trigonoides, Adacna plicata, Cerastoderma lamarcki, Dreissena polymorpha, Dr. rostriformis, Mytilaster lineatus etc.), high crustaceans
(Corophium volutator, Axelboeckia spinosa, Niphargoides compressus, Corophium chelicorne, Paramysis ullskui, etc),
and worms
(Manajunkia caspica).
Hypanis angusticostata is of great importance for the development of food supply
for benthophagous fish species in the Northern Caspian.
It is a favorite food organism of roach and Russian sturgeon.
Importance of species to bioresources production of the Caspian Sea
Commercial characteristics of species, catches. N/A
Fishing gears and fishing zones. N/A
Impact of fisheries on the population status
Human impact/Threats. N/A.
Conservation measures. N/A
References
Galperina, G.E. 1972. On the problem of reproduction of food supply for fish in the Northern Caspian (mollusks).P.p. 37-38. In: TSNIORKH Book of Abstracts. Astrakhan (in Russian).
Galperina, G.E. 1976. Breeding of bivalve mollusks (Bivalvia) in the Northern Caspian. P.p. 1-29. In: Author�s Abstract. Moscow (in Russian).
Karpevich, L.F. 1946. Relationships between some species of the family Cardidae and saline conditions in the Northern Caspian. USSR AS Papers, Vol. 54, 1: 73-75 (in Russian).
Maximova, L.P. 1953. Biology of Hypanis angusticostata in the Sea of Azov. Moscow. P.p. 1-9 (in Russian).
Mordukhay-Boltovskoy, F.D. 1978. Composition and distribution of the Caspian fauna according to present data. In: Elements of aquatic ecosystems. USSR AS Proceedings. 22: 100-139. Moscow (in Russian).
Ossadchikh, V.F. 1967. Seasonal dynamics of the Northern Caspian bivalve mollusks. CaspNIRKH Proceedings. 23: 80-90 (in Russian).
Sanina, L.V. 1975. Preliminary estimation of nutritional requirements of Northern Caspian mollusks, filter feeders. VNIRO Proceedings. 107: 43-46 ( in Russian).
Sayenkova, A.K. 1951. Seasonal changes in the benthos in the zone of summer feeding of roach in the Volga-Caspian region. VNIRO Proceedings. 18: 171-177.
Yablonskaya, E.A.1971. Feeding of benthic invertebrates and trophic structure of the benthos of the Caspian, Azov, and Aral Seas.P.p. 3-44. In: Scientific report on topic 13: Utilization of food resources and trophic relations in the southern seas. Moscow. VNIRO Press (in Russian).
Compiled by:
L.V. Malinovskaya (Caspian Fisheries Research Institute, Astrakhan, Russia)
Acknowledgements:
The author is grateful to Dr. A.A. Polyaninova for valuable comments.