
Taxonomic description of species
The thick-clawed crayfish has a carapace with small tubercles on the sides. The rostrum is tapering at the eyes and its pointed apex does not reach the end of squamules at the antennae. Isosceles pointed pleurae of the third segment of the abdomen end in one or two small spines. The claw is thick and broad. Digits of the claw are shorter than in the Caspian long-clawed crayfish and never join each other throughout their length. The inner margin of the fixed side of the claw always has an emargination limited by two tubercles. On the inner margin of the flexible side of the claw there may be found a tubercle. The outer posterior angle of the antenna squamule has no sharp spines. The carapace is smooth with hardly noticeable spines. The cephalothorax is mostly violet and orange in color. The upper part of the claws is green. The junctions are bright yellow. The ends of the claws are bright red or wine-colored. The carapace may be covered with small yellow spots (Rumyantsev, 1974).
Intraspecific forms. Unknown
Related forms. Unknown
Distribution of species within the Caspian Sea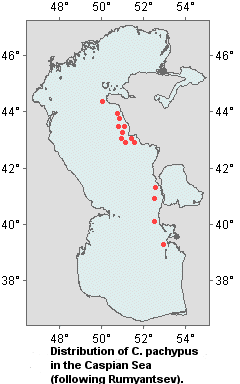
The thick-clawed crayfish occurs north of the Tyub-Karagan Bay. Its mass congregations are recorded at Sagyndyk Cape. The thick-clawed crayfish is most abundant at Melovoy Cape on rocky ridges that extend as far as Peschanyi Cape. It is abundant in the Bay of Alexander Bekovich-Tcherkassky especially near stony islands (Rumyantsev, 1974).
Commercial stocks of crayfish were recorded in the eastern shelf zone from Duldulat Mountain to Kara-Bogaz-Gol (Ushivtsev, 1981), in the Bay of Alexander Bekovich-Tcherkassky (Ushivtsev, 1986), in Kazakh, Krasnovodsky and Turkmen Bays (Ushivtsev, 1998).
During investigations undertaken at offshore banks, crayfish were found at Zhdanov Bank, the average density of the population was 0.02-0.06
ind./m2, at Livanov Bank 0.05-0.08 ind./m2. A lot of crayfish
C. pachypus were found at the nameless bank 20 miles north-west of Livanov Bank. The average density of the population was 1 ind./m2 ranging from 0.5 to 3
ind./m2. The high biomass of red algae and Mytilaster was recorded at that bank (Ushivtsev, Kamakin, 2000).
Status as per International Red Data Book. Not defined
Status as per National Red Data Books. Not defined
First record for the Caspian Sea. The thick-clawed crayfish in the Caspian Sea was first recorded by Rathke in 1837.
Redescription of species. In the "Inventory of Freshwater Invertebrates of Russia" (Alexeyev, 1995)
General characteristics of species
Ecological-taxonomic group. Zoobenthos
Origin. Caspian firth relict
World distribution. Ponto-Caspian endemic
Biotope. Mainly, solid bottoms
Migration. It leads a sedentary life and, as a rule, does not perform long-distance migrations. The extent of seasonal migrations does not exceed 1 km. Tagged crayfish in most water-bodies did not move more than 300 m from the place of their release. Local migrations of crayfish were recorded at the eastern coast of the Middle Caspian where they moved within the limits of the
coastal shallow water zone with depths ranging from 1 to 30 m (Rumyantsev, 1974).
Daily feeding migrations of crayish at a distance of 250-300 m were observed.
Relation to abiotic environmental factors
Relation to salinity. Brackishwater stenohaline species
Water salinity at the southern border of its range is 13�, at the northern -
12�.
Relation to temperature. Stenothermic cold-water species
C. pachypus can not withstand sharp increases in water temperature (Cherkashina, 1974). This may account for crayfish distribution mainly in the northern part of the shelf zone from Tyub-Karagan Cape to Zhilandy Cape where water rarely warms up to
200C in summer. The thick-clawed crayfish in these areas lives mostly at a depth less than 10 m at a water temperature
16-190C. Crayfish are not seen in deeper waters which is attributed to unfavorable temperature regime because of frequent upwelling when water temperature may change by
100C and more for several hours. High densities of thick-clawed crayfish occur at a depth of 15-20 m in southern areas where upwelling is not recorded. The highest densities were noted at a depth of 25-30 m at the most southern point where they were seen (the area of Livanov Bank). Thus, the thick-clawed crayfish prefers rocky bottom with water plants and avoids too warm water (more than
22-260C) and areas where sharp declines in temperature occur.
Vertical distribution. Eurybathic species
Depths from 20 to 100 m are sparsely inhabited. The population density is within 0.01-0.05 ind./m2 there except for Livanov Bank where the density is 3 ind./m2 at depth 15-60 m.
The population density increases to 0.05-0.1 ind./m2 at a depth of 10 m (as compared to deeper waters). The long-clawed crayfish dominates the population there while the thick-clawed crayfish occupies only small areas.
The major stocks of crayfish occur at a depth less than 10 m. The long-clawed crayfish forms the basis of the population while the thick-clawed crayfish occurs only in the area of Mangyshlak. The density is 0.15-0.30
ind./m2.
Relation to oxygen conditions. Crayfish are very sensitive to oxygen conditions and may die during the periods of suffocation. According to
V.D. Rumyantsev (1974), the thick-clawed crayfish mortality was observed at average oxygen content of 2.23 mg/l and water temperature
110C.
Oxygen content in the areas inhabited by thick-clawed crayfish is not below 6.1 mg/l (saturation is 87.5%) (Cherkashina, 1974). The presence of typical marine species of benthic water plants in these areas (Kireyeva, Shchapova, 1957) suggests good aeration.
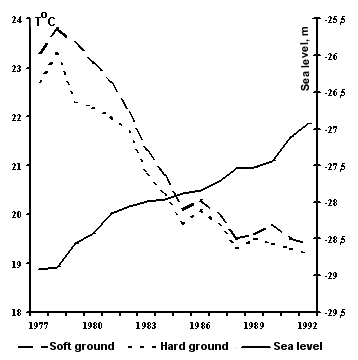
Relation to fluctuations of the sea level. Abundance trends of thick-clawed crayfish on soft grounds were studied in the Bay of Alexander Bekovich-Tcherkassky in 1983. The population density in the late
1980-s reached commercial values there. Crayfish settled in burrows in the sea grass Zostera nana on the bottom covered with sand or silt. This is a unique phenomenon that was not seen earlier as thick-clawed crayfish biotopes occurred only on hard stony grounds. This phenomenon was noted during the period of sea level rise.
According to our observations during 1977-1992, the mean water temperature in the bay in the warmest month, August, dropped from
230C to 21-190C. That was observed during the period of sea level rise. The thick-clawed crayfish was not seen at such biotopes in more southern areas (Kazakh, Krasnovodsky Bay) where water temperature in August is above 25-280C.
Mean water temperature in the Bay of Bekovish-Tcherkassky
in summer 1977-1992 and sea level fluctuations.
Feeding
Feeding type. Heterotrophic
Feeding behavior. Collecting from the bottom
Food spectrum. The mollusk Mytilaster dominates thick-clawed crayfish diet. The crayfish hardly uses higher plants, consumes green algae
insignificantly, but feeds on red algae to a greater extent than the long-clawed crayfish (Cherkashina, 1972). The highest densities of the thick-clawed crayfish were recorded on stony grounds in the thickets of red algae Polysiphonia violacae and Polysiphonia elongata.
Supply of food. The thick-clawed crayfish occurs mainly on hard grounds at a depth not less than 10-20 m. Its congregations are recorded in areas with high benthos biomass where Mytilaster prevails ranging from 100 to 1000
g/m2. Red algae also occur there in large amounts (Cherkashina, 1972).
Quantitative characteristics of feeding. The feeding activity of both crayfish species depends on season. According to our observations in spring, out of 86 long-clawed and 14 thick-clawed crayfish sampled, 48 long-clawed and 6 thick-clawed crayfish (56% and 43%, respectively) had filled stomachs. In summer this number decreased to 45% and 33%, respectively. In autumn all crayfish sampled had filled stomachs.
Reproduction
Reproduction type. Sexual
Reproduction areas. The eastern shelf of the Middle and Southern Caspian
Terms of reproduction. Fishermen say that the crayfish inhabiting the Krasnovodsky Bay �lies down on eggs� in February. In early April 1988 females of the thick-clawed crayfish were lifted to the surface from the depth of 30 m at the shoals of the Southern Caspian. Of 73 specimens sampled, 67 showed signs of recent mating. Water temperature at the biotope was
11.40C.
According to N.Ya. Cherkashina (1970), mating of Caspian crayfish occurs between November and January. V.D. Rumyantsev (1974) noted that the Volga-Caspian crayfish have an extended mating season from January to March. During our investigations, C. pachypus females with signs of recent mating (spermatophores on pleopods) occurred in the Bay of Bekovich-Tcherkassky in early April. Of 93 females of P. eichwaldi sampled in Kara-Bogaz-Gol during February 21-23, 1987, 81 specimens exhibited signs of recent mating.
Fecundity. 15-20 days after mating the female begins to fertilize eggs. She bends her abdomen to cephalothorax and releases the secret dissolving spermatophores into the chamber formed in this way. Then she lays eggs that are fertilized passing through seminal fluid and become attached to pleopods. The female supplies eggs with fresh water constantly moving her pleopods and walking legs thus providing favorable conditions for egg incubation (Rumyantsev, 1974).
The minimum length of mature females C. pachypus is 5.5 cm. But such females are not abundant (5-7% of the size group) (Rumyantsev, 1974).
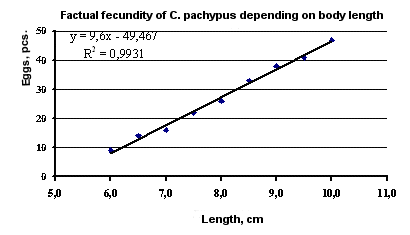
In the second ten-day period of April 1989, the factual fecundity of crayfish at the beginning of incubation was studied in the Bay of Bekovich-Tcherkassky.
C. pachypus abundance in the bay is very high. Egg-bearing females 5.5-6 cm in length accounted for 15-20% of the size group. Such females had 3-15 eggs each. P. eichwaldi females 6-6.5 cm in length at that biotope had 25-30 eggs on their abdomens. The number of females without eggs decreased markedly in subsequent size groups of both species. Such individuals more than 7 cm in length accounted for less than 5%.
The factual fecundity of crayfish correlates with their size. The results of the analysis of 209 females of
C. pachypus are presented:
Limiting factors. Upwelling that adversely affects the development of crayfish eggs may be referred to limiting abiotic factors.
Life history and development
Life history stages. In the process of egg hatching, egg membranes break due to the contraction of embryo muscles. Young crayfish hang on hyaline threads that break 2-3 days later and young crayfish cling to egg membranes by claws with pointed hooks. Larvae are almost immobile and use the yolk under their dorsal scutellum of cephalothorax as food. Young crayfish are molting for about a week and then change to exogenous feeding. The second molt takes place 8-10 days after the first one. By that time young crayfish leave their mother (Rumyantsev, 1974)
Relation to environmental factors. Water temperature and oxygen conditions are of great importance for the development of young crayfish.
Age of maturity. The thick-clawed crayfish becomes mature in the third year of its life.
Thermal conditions of development. According to our observations, eggs of Caspian crayfish hatch at different time. In late June young crayfish appear in Kara-Bogaz-Gol at a water temperature of
19-210C. Young crayfish C. pachypus appear in the Bay of Bekovich-Tcherkassky and coastal waters of Mangyshlak later than anywhere else in early July at a temperature
18-200C. Females with their brood on the abdomen occurred sporadically until early August. The retarded development of larvae is thought to be due to upwelling often recorded there.
Quantitative characteristics of growth. Not studied
Structural and functional population characteristics
Sex ratio. The results of observations show that the reproductive potential of Caspian crayfish varies widely especially during the period of sea level rise.
Various factors affect the population abundance. Upwelling is an abiotic factor that causes a decline in water temperature which adversely affects reproduction (the development of eggs and larvae is delayed). Because of heavy storms, mass mortality of egg-bearing females, i.e. the part of the population most valued for reproduction, was observed. In still summer nights oxygen deficiency occurred in the bottom layers of shallow water bays overgrown with macrophytes which caused crayfish mortality.
Sex ratio in crayfish populations was recorded to change affecting reproductive potential. According to our observations, males of long-clawed crayfish in the Bay of Bekovich-Tcherkassky preferred to settle on hard rocky grounds. The ratio of females to males sampled was 1:2.5 for
P. eichwaldi and 1:5 for
C. pachypus. The ratio of females to males on soft grounds in the same area was 1.5:1. On the whole, the number of males in the population of
P. eichwaldi was 1.4-fold greater than that of females. The number of males in the population of
C. pachypus was 2.6 times larger than that of females. These data were obtained in 1992 when sea level was +199 cm.
From literature (Rumyantsev, 1974; Cherkashina, 1976), the ratio of females to males obtained from the observations during the period of sea level �28.8 m (from Baku tide-gauge), which corresponds to the level +5 cm, was 1:1 for
P. eichwaldi and 1:5 for C. pachypus. In 1992 when the sea level was +199 cm, the reproductive potential of
P. eichwaldi decreased because of a 1.4-fold increase in the number of males. At the same time the reproductive potential of
C. pachypus increased due to 1.9-fold increase in the number of females. This is reflected by the total number of crayfish in the bay. During the period of observations (1978-1992), the number of long-clawed crayfish decreased by a factor of 6.2, that of the thick-clawed crayfish increased 15-fold.
In 1978 when the sea level was +4 cm, the total number of females of P. eichwaldi in the bay was estimated at 3.68 million. If the mean number of larvae per female is 100, the reproductive potential of the population is 368 million larvae. In 1992 when the sea level was +199 cm, the total number of females of
P. eichwaldi in the bay decreased to 1.2 million and the reproductive potential was reduced to 120 million larvae, i.e. to 1/3 of that in 1978.
The total number of females of C. pachypus in the bay in 1978 (sea level +4 cm) was estimated at 117450 individuals. If the mean number of larvae per female is 15, the reproductive potential is 1.7 million larvae. In 1992 when sea level was +199 cm, the total number of females in the bay increased to 1.8 million. At that, the reproductive potential increased 15 times reaching 27 million larvae.
Sex ratio of P. eichwaldi in the Kazakh Bay varied depending on biotope and season. Females preferred coastal warm waters and hard grounds �stones-sand�. The ratio of females to males there was 2:1. Males were in the majority at the biotope �sand-zostera� and the ratio was 1:2. Sex ratio 1:1 was recorded at the biotope �silt-zostera�. The same sex ratio (1:1) was determined for the entire crayfish population in the Kazakh Bay. It should be noted that during the period of observations in that area crayfish abundance was most stable and sea level fluctuations did not affect the reproductive potential of the population.
Over 11 years of observations (1981-1992) in Kara-Bogaz-Gol, the sea level has risen by 122 cm. During that period the number of thick-clawed crayfish increased eightfold and the ratio of females to males was 1:2.5, similar to that in the Bay of Bekovich-Tcherkassky. The long-clawed crayfish abundance in Kara-Bogaz-Gol increased 1.5-fold with female to male ratio 1:1.
Summarizing data, it may be concluded that sea level rise affects the reproductive potential of crayfish populations changing the ratio of females to males. But sea level fluctuations in different areas have various effects.
Age-size structure. At present there are no methods for reliable estimation of the age of crayfish. The size-age structure of thick-clawed crayfish is not studied.
Quantitative characteristics.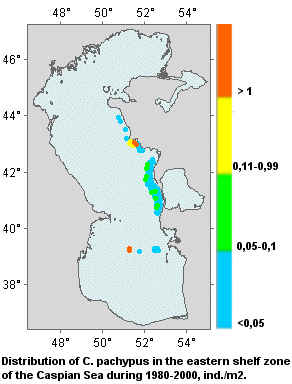 The thick-clawed crayfish is most abundant in the following areas: not far from
villages Yeralievo, Bektash, Aim , in the Bay of Bekovich-Tcherkassky. The population density in the coastal shelf zone at a depth of 10 m is 0.05-0.1
ind./m2. The crayfish density in the bays at biotopes less than 10 m in depth is 0.5-1.0
ind./m2 (Sokolsky, Ushivtsev, Kolmykov, Mikouiza, 1999).
The thick-clawed crayfish is most abundant in the following areas: not far from
villages Yeralievo, Bektash, Aim , in the Bay of Bekovich-Tcherkassky. The population density in the coastal shelf zone at a depth of 10 m is 0.05-0.1
ind./m2. The crayfish density in the bays at biotopes less than 10 m in depth is 0.5-1.0
ind./m2 (Sokolsky, Ushivtsev, Kolmykov, Mikouiza, 1999).
Population trends. The absence of commercial harvest and reduced predator pressure (due to the decrease in the number of fish feeding on crayfish: beluga sturgeon, catfish, sea zander) resulted in the increase in crayfish stocks.
Interspecific relations
The abundance of crayfish in the Caspian Sea is controlled by the predation of sturgeons, sea zander, catfish, Caspian
seal. Beluga sturgeon feed on crayfish most actively. Up to a hundred crayfish were found in stomachs of some individuals. Sometimes, crayfish account for 40% of the catfish diet. Birds also consume a lot of crayfish. Crayfish were found in stomachs of crows, rooks, cormorants, terns, geese, ducks (Rumyantsev, 1974).
Impact on the ecosystem
Pontastacus eichwaldi Bott is an autochthonous species for the Caspian Sea ecosystem and one of the elements of biocenosis. On the one hand, it is a consumer of food resources, on the other hand, it serves as food for some fish, birds and mammals.
Importance of species to bioresources production of the Caspian Sea
Economic significance of species. Valued food that may be referred to seafood delicatessen.
Commercial characteristics of species, catches. Caspian long-clawed crayfish reach 17.0 cm in length and 180.0 g in weight. Their average length varies from 12.0 to 13.0 cm with weight 80.0- 90.0 g. First information regarding crayfish harvesting in the Caspian Sea refers to the early
20-th century. Some 4-6 million crayfish were caught in the Krasnovodsky Bay for a year (Shavrov, 1910). Even when harvested irregularly, not less than 30 000 crayfish were caught at the coast of Mangyshlak annually (Rumyantsev, 1974). During the 1920s-1940s crayfish were harvested occasionally. Since 1959 the catch has been based on crayfish harvested in the Krasnovodsky Bay. Turkmenistan�s catches of crayfish varied between 100 and 1200 metric centners per year. In 1969 the commercial harvest was halted because of establishing an ornithological reserve in the Krasnovodsky Bay. In the 1980s annual catches of crayfish amounted to some 5 tonnes. The thick-clawed-crayfish stock in 2001 reaches some 500 tonnes.
Fishing gears and fishing zones. Crayfish traps are traditionally used for catching crayfish (crayfish traps designed by
P.G. Agachev (1953). They have the form of a truncated cone covered with fine-meshed net with a top inlet. A flapper made of fine-meshed net prevents crayfish from escape. Promising areas in terms of commercial harvest of crayfish are the bays: Kazakh, Turkmen,
Bekovich-Tcherkassky, Kianly, Kara-Bogaz-Gol.
Impact of fisheries on the population
At the present time commercial harvest of crayfish is not conducted. Some anglers catch crayfish, but their catches are not large and can not impact the population status. Data on the volume of crayfish harvest in Iranian waters are not available.
Human impacts/Threats. As crayfish are particular to water quality, a decline in their stocks may be expected because of sea pollution from oil field development and exploitation at sea.
Conservation measures
- Application of environmentally safe technologies for oil production and transportation at sea.
- Extension of crayfish productive biotopes through construction of artificial reefs in the areas lacking shelters
necessary for molting young and adult crayfish.
- Stocking artificially constructed and natural biotopes with young crayfish including farmed juveniles.
References
Agachev, P.G. 1953. Modified crayfish trap. Rybnoye Khozyaistvo, 8: 47-48 (in Russia).
Alexeyev, V.P. 1995. Inventory of freshwater invertebrates. St.Petersburg, Nauka. Vol.2, p.178 (in Russia).
Bokova, E.N. 1948. Freshwater crayfish of the Caspian Sea. Rybnoye Khozyaistvo, 9: 32-37 (in Russian).
Cherkashina, N.Ya. 1970. Crayfish distribution in the Krasnovodsky Bay (the Caspian Sea). VNIRO Young Scientists Proceedings, 4: 53-59 (in Russian).
Cherkashina, N.Ya. 1970. On crayfish (Astacidae) reproduction at the south-eastern coast of the Caspian Sea. Hydrobiological J., 6, 4: 104-106 (in Russian).
Cherkashina, N.Ya. 1972. Feeding of Astacus leptoductylus Bott, Astacus pachypus Rathke in the Turkmen waters of the Caspian Sea. VNIRO Proceedings, V.90: 55-71 (in Russian).
Cherkashina, N.Ya. 1974. Biology of Astacus leptoductylus Bott and Astacus pachypus Rathke in the Turkmen waters of the Caspian Sea. VNIRO Proceedings, 90: 55-71 (in Russian).
Cherkashina, N.Ya. 1975. Distribution and stocks of crayfish of the genus Astacus (Crustacea, Decapoda, Astacidae) in the Turkmen waters of the Caspian Sea. VNIRO Proceedings, 108: 177-184 (in Russian).
Cherkashina, N.Ya. 1976. Distribution and biology of the thick-clawed crayfish in the Turkmen waters of the Caspian Sea. Zool. J., 55, 4: 602-606 (in Russian).
Cherkashina, N.Ya. 1968. On biology and harvest of crayfish at the Turkmen coast. P. 164. In: Elaboration of biological principles and biotechnique of sturgeon fisheries development in the USSR water bodies. Ashkhabad (in Russian).
Cherkashina, N.Ya. 1974. Biology of Astacus leptoductylus Bott and Astacus pachypus Rathke in the Turkmen waters of the Caspian Sea. VNIRO Proceedings, 90, 5: 70-83 (in Russian).
Grimm, O.A. 1877. The Caspian Sea and its fauna. Proceedings of the Aral-Caspian Expedition, 2, 2, 105 p. (in Russian).
Grimm, O.A. 1877. The Caspian Sea and its fauna. Proceedings of the Aral-Caspian Expedition, 2, 2, 105 p. (in Russian).
Kessler, K.F. 1875. Russian freshwater crayfish. Proceedings of the Russian Entomological Society, Vol. 8, 3-4: 355-423 (in Russian).
Kireyeva, M.S. and T.F. Shchapova. 1957. Materials on the systematic composition and biomass of algae and higher water plants of the Caspian Sea. Proceedings of the Institute of Oceanography, 23: 125-137 (in Russian).
Rumyantsev, B.D. 1974. Freshwater crayfish of the Volga �Caspian. Moscow. Food Industry. 84 p. (in Russian).
Shavrov, N.I. 1910. Crayfish harvest in the Krasnovodsky Bay. Vestnik Rybprom. 7: 293-308. Moscow (in Russia).
Sokolsky, A., V. Ushivtsev, E. Kolmykov, A. Mikouiza 1999. Influence of sea level fluctuations on wild crayfish population in the Caspian Sea. P.P. 655-664. In: Proceedings of the 12th IAA Symposium. Augsburg. Germany.
Starobogatov, Ya.I. 1995. Crustaceans. Inventory of Freshwater Invertebrates in Russia. V.2: 177-180 (in Russian).
Suvorov, E.K. 1915. On crayfish harvest along the eastern coast of the Caspian. P.p. 33-39. In: Materials for Knowledge of Russian Fishery. Vol. 4, 15 (in Russian).
Ushivtsev, V.B. 1981. Assessment of crayfish stocks in the Caspian Sea. Rybnoye Khozyaistvo, 4: 50-51 (in Russian).
Ushivtsev, V.B. 1986. Distribution and stocks of crayfish in the Kazakh waters of the Caspian Sea. P.121. Book of Abstracts of the 19th Scientific Conference: Biological Principles of Fisheries in Water Bodies of Central Asia and Kazakhstan (in Russian).
Ushivtsev, V.B. 1992. Commercially harvested marine species. P.p. 98-99. In: Scientific principles of regional distribution of commercially exploited species of the Caspian Sea. BIVTS Kaspryba (in Russian).
Ushivtsev, V.B. and A.M. Kamakin. 2000. Crayfish of the Southern Caspian (Crustacea: Decapoda, Astacidae): distribution, biotope characteristics. Scientific Bulletin of the Caspian Floating University, 1: 157. CaspNIRKH Press. Astrakhan (in Russian).
Ushivtsev, V.B., A.M. Kamakin, E.V. Kolmykov, A.Yu. Goncharov. 1994. The status of crayfish stocks (Crustacea, Decapoda, Astacidae) in the eastern shelf zone of the Caspian Sea during the period of sea level rise. P. 89. In: Ecosystems of the seas of Russia under anthropogenic impact including fishery (in Russian).
Zehnder, H. 1934. Uber die Embrionalentwicklung des Flusskrebses. Acta zool. Bd 15: 9-16.
Compiled by:
V.B. Ushivtsev (Caspian Fisheries Research Institute, Astrakhan, Russia)