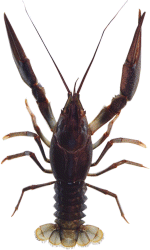
Taxonomic description of species
The body of higher crayfish consists of three parts: cephalon, thorax and abdomen. The cephalon consists of five closely merged segments and an acron. Eyes are on the head linked to it with flexible pedicles. In the anterior margin of the cephalon there is a rostrum. The carapace accretes closely to all eight segments of the thorax.
The abdomen is dorsally and ventrally flattened and consists of six segments and an anal segment, telson. The telson represents a plate with small spines located at the ends.
There are three pairs of biramous abdominal appendages (pleopods) (the first two pairs in males turned into the copulative organs, gonopods that are not seen in females) and a pair of urapods. The latter together with the telson forms a tailfan (Fomichev, 1986).
The species Pontastacus eichwaldi has the following distinction: the margins of the anterior part of the telson (in front of the lateral spines) are not parallel and converge backwards. The terminal lobes of gonopods 1 of the male are markedly different in length (Starobogatov, 1995).
Males and females have pronounced sexual distinctions. Genital pores Sexual openings of males are located at the base of the fifth pair of thoracic appendages. The breadth of the abdomen is equal to that of the cephalothorax. The claws are large. Genital pores Sexual openings of females are located at the base of the third pair of appendages.
The abdomen is broader than cephalothorax. The claws are far smaller.
Age Variability. 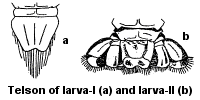 A larva hatching from egg membranes (larva-I) is not like adult crayfish in appearance. It has a large cephalothorax, the telson is oval, the rostrum is bent. Claws are very pointed with hooks at the ends. After the first molt, larvae begin to resemble adult crayfish, but differ from them in the shape of the telson that consists of one plate and is rounded in form (larva-II)
A larva hatching from egg membranes (larva-I) is not like adult crayfish in appearance. It has a large cephalothorax, the telson is oval, the rostrum is bent. Claws are very pointed with hooks at the ends. After the first molt, larvae begin to resemble adult crayfish, but differ from them in the shape of the telson that consists of one plate and is rounded in form (larva-II)
After the second molt, larvae turn into fully developed young crayfish. They can be easily distinguished by the structure of the telson that consists of three plates arranged in a fan.
Local Variability. Crayfish inhabiting different areas differ in some qualitative characteristics: coloration, density and shape of spines on the carapace, length-width relation of the claw.
Intraspecific Forms.
Dnestrovsky and Kuchurgansky firths are inhabited by P.e.bessarabicus Brodsky, 1981 (Starobogatov, 1995).
Related Forms.
Allied species are widely distributed in Europe: P.intermedius Brodsky,
1981;
P.leptodactylus (Eschscholtz, 1823); P.salinus (Nordmann.
1842);
P.pylzovi (Skorikov, 1911); P.kessleri (Schimkewitsch, 1884); P.cubanicus (Birstein et
Winogradow, 1934);
P.danubialis Brodsky, 1981.
Distribution of species within the Caspian Sea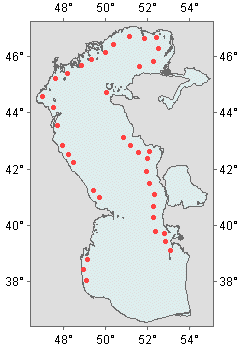
It is distributed throughout the coast of the Middle and Southern Caspian. The abundance is higher in the eastern shelf zone from the Mangyshlak Peninsular to Krasnovodsky Bay. High densities are not recorded along the western coastline. It occurs throughout the Northern Caspian, but the densities are not high except for the Kizlyarsky Bay and the mouth of the Volga River (Rumyantsev, 1974).
Status as per International Red Data Book.
Not defined.
Status as per National Red Data Books. Not defined.
First record for the Caspian Sea. E. Eichwald, 1838
Redescription of species. R. Bott, 1950; Ya.I. Starobogatov, 1995
General characteristics of species
Ecological taxonomic group. Zoobenthos
Origin. Autochthonous Caspian species
Distribution.
Presently, the species inhabits the Caspian Sea, Volga River delta, western steppe side waters.
Biotope.
Crayfish inhabit the areas of the Northern Caspian with the bottom covered with silt, sand and silt, shell rock. At the coast of the Middle and Southern Caspian, crayfish live on stony, sludgy grounds, in areas with much cockleshell and debris of limestone slabs, as well as in moderate thickets of red and green algae.
Migration.
Crayfish lead a sedentary life and, as a rule, do not perform long-distance migrations. The extent of their seasonal migrations does not exceed 1 km. Local migrations of crayfish were recorded at the eastern coast of the Middle Caspian. In autumn crayfish migrations occur in the avant-delta of the Volga River (Rumyantsev, 1974).
Relation to abiotic environmental factors
Relation to salinity. Brackishwater euryhaline species.
Water salinity ranges from 13o/oo at the southern border of the area to 0o/oo (fresh water) at the north, in the Volga River delta.
Relation to temperature. Eurythermic species
In winter it can withstand temperatures below 1.00C, in summer endures a rise in temperature to
300C.
Vertical distribution.
Eurybathic species
Pontastacus eichwaldi Bott occurs at depths of 0.5-100 m. The highest densities were noted in shallow water creeks at depths from 2 m to 10 m.
Relation to oxygen conditions.
It can endure a decrease in oxygen content to 1mg/l.
Relation to fluctuations of the sea level. Sea level rise resulted in a decrease in population number by a factor of 6.2 in the Bay of Bekovich-Tcherkassky. The number of the population remained the same in the Kazakh Bay and showed a 1.5-fold increase in Kara-Bogaz-Gol (Ushivtsev, Kamakin, Kolmykov, Goncharov, 1994).
Feeding
Feeding type. Heterotrophic
Feeding behavior.
The long-clawed crayfish gets food by gathering food organisms from the bottom of the reservoir. It grasps them with two pairs of walking legs and puts small pieces into the mouth crushing them by three pairs of gnathopodites (two pairs of lower and one pair of large upper ones moving right-left). If the prey is large in size, it is first torn into pieces
with the claws. In the stomach the food is ground by three gastroliths and then passes through specifically arranged villi that strain off large fractions that are then ejaculated.
Food spectrum.
Ascillaria, Zostera and Cladophora are vegetable components most frequently used by crayfish. Larvae of midges (Chironomidae), small
mollusks, crustaceans, Nereis and Mytilaster are common animal components. Young crayfish diet contains a lot of Ostracoda
(Kurashova, Rumyantsev, 1970). The proportion of animal and vegetable components in young crayfish diet changes, as they grow, in the following way:
| Young crayfish length, mm |
Proportion of animal food, % |
| 12-16 |
97.9 |
| 16-21.5 |
41.7 |
| 21.6-23 |
47.4 |
| 23-30 |
51.2 |
Beginning from one-year old age, the diet of young and adult crayfish is the same.
Supply of food. Information is not available
Quantitative characteristics of feeding. Daily food consumption of one individual makes up 1%-5% of its body weight (Tsukerzis, 1970;
Cherkashina, 1972). The frequency of food intake by males is once every two days, that by females is once every three days. Females consume 0.78 g, males consume 0.52 g of
food per intake (Tsukerzis, 1970).
When speaking of feeding activity of freshwater crayfish, seasonal and daily aspects should be mentioned. Maximum activity of crayfish is recorded during the periods prior to reproduction and molt as well as after molt. Minimum activity is noted during the egg-bearing season and hatching as well as during molt and calcification of the carapace. From
V.D. Rumyantsev�s (1974) observations, the main factor affecting daily rhythms of long-clawed crayfish feeding is the light. Crayfish feed day and night in turbid water, in clear water - only at night.
Reproduction
Reproduction type.
The Caspian long-clawed crayfish is a dioecious animal with external fertilization. Eggs develop in follicles. When follicles mature, they burst and an egg gets into the ovary and then enters the oviduct. Crayfish eggs are centrolecithal. 15-20 days after spermatophores are introduced onto the female, she begins to lay eggs. The female bends her abdomen to the cephalothorax thus making a closed space in which the glandular fluid is secreted. It dissolves the agglutinating substance of spermatophores. After that, eggs are taken out and fertilized. On the lateral sides of female sternites, there are excretory ducts of cement glands that secrete the mucous fluid hardening as threads and sticking eggs to swimming legs of the female (Zehnder, 1934).
Location of reproduction.
There are no specific areas of reproduction that occurs at species habitats.
Timing of reproduction.
The season of mating and egg laying of Caspian crayfish is extended (from February to May). Egg hatching usually occurs in June-July.
Fecundity.
The absolute fecundity of Caspian long-clawed crayfish averages 276 eggs, factual fecundity is 193 eggs. Factual fecundity of females correlates with their size (correlation coefficient is
0.75). The equation describing relation between parameters of body length and factual fecundity is as follows:
y=64.7275-18.91x+2.478x2 (Rumyantsev, 1974).
Limiting factors.
The main factors affecting crayfish reproduction are: temperature conditions, substrate (shelters available), water quality (pollution).
Life history and development
Life-history stages.
Embryonic development of Caspian crayfish lasts for 3.5-5 months. Eggs hatch and larvae hang on hyaline threads that break 2-3 days later. A young crayfish clings to the egg membrane by claws with pointed hooks. At that stage the larva does not resemble an adult crayfish. It has a large cephalothorax, the rostrum is bent, the telson is oval in form. The larva is almost immobile and uses the yolk under its dorsal scutellum of the cephalothorax as food.
Larvae are molting for about a week and then transit to exogenous feeding. At that time, they assume the structural features of adult crayfish. In the second-third year of their life, crayfish begin producing progeny.
Relation to environmental factors.
The initial development of embryos occurs at a temperature not more than 6.0-9.50C. Egg hatching is observed at
18.0-25.00C. Young crayfish endure temperatures ranging from 20.0 to
30.00C. The temperature they tolerate in the course of adaptation to natural conditions has a lower
(10.00C) and upper (34.00C) limits. Young crayfish, large in size, are more tolerant of thermal effects, the mean lethal temperature is
39.10C, 38.40C � for smaller juveniles. (Kolmykov, 2001).
Mortality caused by an extreme thermal effect occurs due to increasing K+ concentration in the hemolymph and spontaneous activity of neurons (Bowler et al., 1973).
Young Caspian long-clawed crayfish living at a salinity 12-13o/oo show a good capacity for osmoregulation and develop physiological mechanisms of protection from hyperhydration, which allow them to survive in fresh water (Kolmykov, 2000). Adult crayfish
Pontastacus eichwaldi Bott living in seawater can hardly endure sharp changes in salt concentration and die when transferred to fresh water.
The optimum pH for crayfish is 6.8-8.2 (Fomichev, 1986).
Age of maturity.
Mature females of the Caspian long-clawed crayfish have a minimum length of 7-8 cm which corresponds to the age of 2 years. Mature females account for 5-7% of this size group.
The number of reproductive females above 8 cm TL increases to 86-94% (Rumyantsev, 1974).
Thermal conditions of development.
Mating, fertilization and laying of eggs occur at a temperature of 7.8-9.50C. Embryos develop at a temperature between
100C and 200C. Hatching occurs at 17.0-24.00C.
Quantitative characteristics of growth.
The length increment of one-summer old crayfish is 0.4 mm in one molt. Young crayfish do not vary markedly in size
until the sixth molt, they differ in body length by 0.2-0.3 mm. Beginning from the seventh molt, the difference in body length increases, young crayfish become more differentiated by their growth rate. By autumn the difference in length between individuals may reach 36.0 mm. Fast-growing individuals may be as long as 63.0 mm, slow-growing ones reach 27.0 mm, the average length - 43.0 mm.
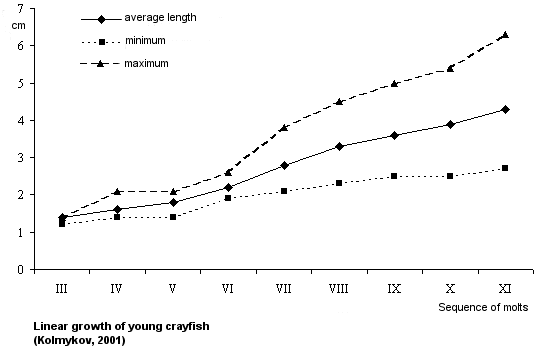
Weight gain in one molt is about 121.0-609.7 mg, 263.7 mg in average. After the sixth molt young crayfish vary in weight considerably. The weight discrepancy before the sixth molt is 30.0 mg, then it increases to 5680.0 mg. By October large individuals reach 6150.0 mg in weight, small ones are 470.0 mg in weight, average Wt - 2160.0 mg.
2160.0 mg.
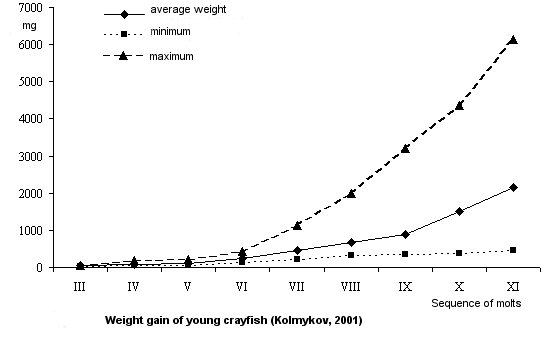
The length increment of two-summer-old crayfish constitutes 2.8 cm per season, weight gain is 8.8 g. By October two-summer-olds reach an average length of 7.6 cm (5.8-10.1 cm) and gain in weight 12.7 g (5.6-32.7 g).
Structural and functional population characteristics
Sex ratio.
The sex ratio is close to 1:1 (Rumyantsev, 1974). Females predominate among small individuals. The proportion of males increases as crayfish grow.
Age-size structure.
At the present time there are no methods for reliable determination of the age of crayfish. It can be calculated from the equation of size and age relation y =
1.596+3.928x-0.238x2.
The average size of the Caspian long-clawed crayfish is 10.5 cm for males and 9.9 cm for females. The maximum size of males is 17.2 cm. The relationship between the size (x) and weight (y) of crayfish is defined by the equation
y=70.28-19.11x+1.5x2, where r=0,9 (Rumyantsev, 1974).
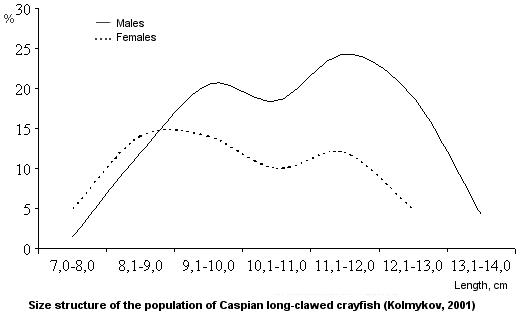
Quantitative characteristics.
In the Caspian Sea basin the long-clawed crayfish is most abundant in the Volga and Ural River mouths, near Yeraliyevo and Bektash Villages,
in the Kianly, Bekovich-Tcherkassky, Kara-Bogaz-Gol, and Krasnovodsky Bays. The density of the crayfish population at a depth of 10 m in coastal areas of the shelf zone is estimated at 0.05-0.1
ind./m2. It reaches 0.5-1.0 ind./m2 at biotopes in bays at a depth less than 10 m (Sokolsky, Ushivtsev, Kolmykov, Mikouiza, 1999). The total stock of Caspian long-clawed crayfish in the Caspian Sea amounts to 690
tonnes.
Population trends.
The absence of commercial harvest and reduced predatory pressure (due to the decrease in the number of fish feeding on crayfish: beluga sturgeon, catfish, sea zander) resulted in the increase in crayfish stocks.
Interspecific relations
The abundance of crayfish in the Caspian Sea is controlled by the predation of sturgeons, sea zander, catfish, Caspian seals. Beluga sturgeon feed on crayfish most actively. Up to a hundred crayfish were found in stomachs of some individuals. Sometimes, crayfish account for 40% of the catfish diet. Birds also consume a lot of crayfish. Crayfish were found in stomachs of crows, rooks, cormorants, terns, geese, ducks (Rumyantsev, 1974).
Impact on the ecosystem
Pontastacus eichwaldi Bott is an autochthonous species for the Caspian Sea ecosystem and one of the elements of biocenosis. On the one hand, it is a consumer of food resources, on the other hand, it serves as food for some fish, birds and mammals.
Importance of species to bioresources production of the Caspian Sea
Economic significance of species.
Valued food that may be referred to seafood delicatessen.
Commercial characteristics of species, catches.
Caspian long-clawed crayfish reach 17.0 cm in length and 180.0 g in weight. Their average length varies from 12.0 to 13.0 cm, weight
- 80.0- 90.0 g. First information regarding crayfish harvesting in the Caspian Sea refers to the early
XX-th century. Some 4-6 million crayfish were caught in the Krasnovodsky Bay annually
(Shavrov, 1910). Even when harvested irregularly, not less than 30, 000 crayfish were caught at the coast of Mangyshlak annually (Rumyantsev, 1974). During the 1920s-1940s crayfish were harvested occasionally. Since 1959, the catch has been based on crayfish harvested in the Krasnovodsky Bay. Turkmenistan�s catches of crayfish varied between 100 and
1, 200 metric centners per year. In 1969 the commercial harvest was halted because of establishing an ornithological reserve in the Krasnovodsky Bay. In the 1980s annual catches of crayfish amounted to some 5 tonnes.
Fishing gears and fishing zones.
Crayfish traps are traditionally used for catching crayfish. They have the form of a truncated cone covered with fine-meshed net with a top inlet. A flapper made of fine-meshed net prevents crayfish from escape.
A flapper made of fine-meshed net prevents crayfish from escape. Prospective areas of commercial crayfish harvest are the bays: Kazakh, Turkmen, Bekovich-Tcherkassky, Kianly, Karshi, Kara-Bogaz-Gol.
Impact of fisheries on the population status
At the present time commercial harvest of crayfish is not conducted. Some anglers catch crayfish, but their catches are not large and can not impact the population status.
Human impacts/Threats.
Industrial pollution and municipalsewage (coastal cities, villages, industrial facilities, ports, etc.) adversely affect the crayfish population. Their stock declines (up to extinction in certain biocenoses) in areas of oil field development and exploitation at sea.
Conservation measures
- Application of environmentally safe technologies for oil production and transportation at sea.
- Extension of crayfish productive biotopes through construction of artificial reefs in the areas lacking shelters necessary to young and adult crayfish for molt.
- Stocking artificial and natural biotopes with young crayfish including farmed juveniles.
References
Brodsky, S.Ya. 1954. Freshwater crayfish (Astacidae) of the Ukrainian SSR, their biology and harvest. Author�s Abstract of the Dissertation. Kiev. 19 p. (in Russia).
Bowler K., Gladwel R.T., Duncan C.J. 1973. Acclimatization to temperature and death at high temperatures in the crayfish Austropotamobius pallipes. In: Freshwater crayfish Pap. The 1st Symp. Austria. Lund:121-131.
Cherkashina, N.Ya. 1972. Feeding of Astacus leptoductylus Bott, Astacus pachypus Rathke in the Turkmenian waters of the Caspian Sea. VNIRO Proceedings, V.90: 55-71 (in Russian).
Fomichev, N.I. 1986. Freshwater crayfish: research methods. Leningrad. 96 p. (in Russian).
Kolmykov, E.B. 2000. Response of young Caspian crayfish to changes in water salinity. Caspian Floating University, Scien. Bull., 1:132-133. Astrakhan (in Russian).
Kolmykov, E.B. 2001. Biological principles of regulation of crayfish (Pontastacus) abundance in the Volga River delta. Author�s Abstract of Dissertation. Astrakhan. 24 p. (in Russian).
Kurashova, E.K., V.D. Rumyantsev. 1970. On the problem of growth and feeding of young long-clawed crayfish. P.p. 26-41. In: Proceedings of the Meeting on the Increase in Stocks and Crayfish Exploratory Fishing. Kiev (in Russian).
Shavrov, N.I. 1910. Crayfish harvest in the Krasnovodsk Bay. Vestnik Rybprom. 7: 293-308. Moscow (in Russia).
Sokolsky, A., V. Ushivtsev, E. Kolmykov, A. Mikouiza 1999. Influence of sea level fluctuations on wild crayfish population in the Caspian Sea. P.P. 655-664. In: Proceedings of the 12th IAA Symposium. Augsburg. Germany.
Starobogatov, Ya.I. 1995. Crustaceans. Inventory of Freshwater Invertebrates in Russia. V.2: 177-180 (in Russian).
Tsukerzis, Ya. M. 1970. Biology of noble crayfish. Vilnius. 205 p.
Tsukerzis, Ya. M. 1989. Freshwater crayfish. Vilnius. 140 p.
Ushivtsev, V.B., A.M. Kamakin, E.V. Kolmykov, A.Yu. Goncharov. 1994. The status of crayfish stocks
(Crustacea, Decapoda, Astacidae) in the eastern shelf zone of the Caspian Sea during the period of a rise in sea level. P. 89. In: Ecosystems of the seas of Russia under anthropogenic impact including fishery (in Russian).
Zehnder, H. 1934. Uber die Embrionalentwicklung des Flusskrebses. Acta zool. Bd 15: 9-16.
Compiled by:
Eugene V. Kolmykov (Caspian Fisheries Research Institute,
Astrakhan, Russia)