ATTACHMENT
4.
Development Mnemiopsis leidyi
population in the Black Sea and
other seas of Mediterranean basin
Tamara Shiganova
In the early 1980s, M.leidyi was
accidentally introduced to the Black Sea. It is a self-fertilizing hermaphrodite,
preadapted to rapid colonization in condition of absence of predators it rapidly reached
very high abundance. In addition M. leidyi has an ability to regenerate from
fragments larger than one quarter of an individual.
By summer-autumn, 1988 it was found
everywhere, at an average biomass of c. 1 kg WW m-2 (40 g WW m-3) and average abundance of
c. 310 m-2 (12.4 m-3) (Vinogradov et al., 1989) (Fig.1). In autumn 1989, the
greatest mean biomass ever in the open sea 4.6 kg WW m-2 (184 g m-3 ) and greatest
abundance, 7,600 m-2 (304 m-3) were measured In spring 1990, abundance was still very
high, but by summer, it began to decrease (Vinogradov et al,1992). This decreasing trend
reversed in September 1994, with an average biomass of 2.7 kg WW m-2 (108 g WW m-3) in the
open sea, but much higher values in inshore waters (maximum 9.7 kg WW m-2, 176 g WW m-3,
average 4.5 kg WW m-2, 180 g WW m-3). A second peak in biomass was observed in spring and
summer 1995 (Fig. 1), followed by a second decrease. In 1998, a third increase in
offshore waters produced an average biomass of 876 g WW m-2 (35 g WW m-3) and an average
abundance of 463 m-2 (18 m-3) (Shiganova, 1998).
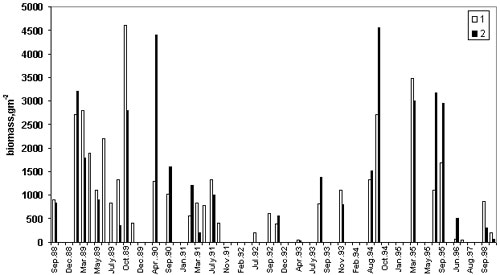
Figure 1. Long-term
variation of M.leidyi biomass in the inshore (1) and offshore (2) the Black Sea
Vertical distribution
M. leidyi mainly lives above the
thermocline (from 0 to 15-25 m) during the warm season. Few large specimens venture below
the thermocline but remain above the pycnocline (60-80 m). In winter, M. leidyi is
found throughout the isothermal layer above the pycnocline, with most of the population
above 50 m.
Seasonal dynamics and factors
controlling population size
M. leidyi, as most
ctenophores, is annual and does not survive Black Sea winters in areas where water
temperatures decrease below 4oC Body size is smaller in spring after a cold
winter , and population size after cold winters is low and most often after cold winter
follows temperate warm summer and population size of M.leidyi keeps rather low
during a year (Fig.2)
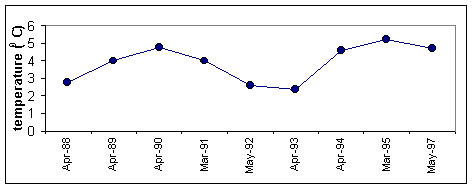
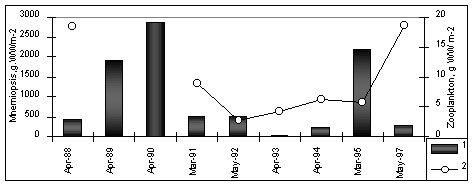
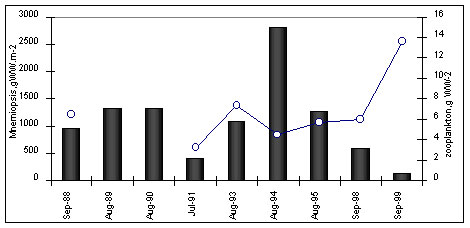
Figure 2. Long-term
variation of M.leidyi biomass (1), zooplankton biomass(2) and average temperature air in
winter (upper panel) and surface water in August (third panel)
Between February and June, overwintering
specimens increase by somatic growth only . By June-July, they reach adulthood. Episodic
reproduction first begins where zooplankton is abundant and temperature higher than 21oC.
Reproduction in inshore waters become
intense in mid July to mid August, and continues until October-November. It requires
temperatures of 23.5-24oC, and peaks at 24.5-25.5oC. Food supply is also critical , and
the main areas of reproduction are inshore waters with abundant
Between February and June, overwintering
specimens increase by somatic growth only . By June-July, they reach adulthood. Episodic
reproduction first begins where zooplankton is abundant and temperature higher than 21oC.
Mean individual weight reaches a maximum in July-August, such that biomass peaks in
August-September. Density, however, peaks in September-November when reproduction is
greatest and the population contains many larvae and small individuals . Reproduction in
inshore waters become intense in mid July to mid August, and continues until
October-November. It requires temperatures of 23.5-24oC, and peaks at 24.5-25.5oC. Food
supply is also critical , and the main areas of reproduction are inshore waters with
abundant mesozooplankton in August-September, the time of the so-called “second peak”
of zooplankton, composed of warm-water species and larvae of benthic organisms
(meroplankton) . However, reproducing ctenophores also spread to the open sea,
where spawning continues.
During October-November, biomass
decreases due to the presence of numerous young individuals (< 3 g WW) and to the death
of large individuals after reproduction . In late October to early November, reproduction
gradually comes to a standstill, at first in the inshore waters, where temperature drops
quickest.
Penetration into the other seas of
Mediterranean basin
M.leidyi from the Black Sea
penetrated into the Azov, Marmara, eastern Mediterranean (Fig.3).
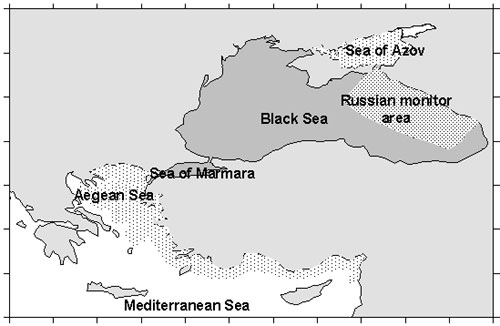
Figure 3.
Distribution M.leidyi in the Mediterranean basin
The Mediterranean is a system of
semi-enclosed seas connected by straits and M.leidyi could penetrated through
straits into the adjacent seas.
Penetration in the Sea of Azov
In 1988 M.leidyi for the first
time was found in the Sea of Azov. M.leidyi can survive there only during warm
seasons, eliminated in autumn when temperature reachs 40C (Volovik et al,
1993).
Penetration in the Sea of Marmara
Probably, M. leidyi penetrated the
Sea of Marmara with the upper Black Sea current through Bosporus in 1989-1990. It now
occurs year-round in the upper water layer. Intense reproduction was recorded in early
October 1992, when mean biomass was 4.2 kg.m-2 (152 g WW m-3) and density 27
m-3 (Shiganova, 1993)
Distribution in the Aegean Sea and in
the eastern Mediterranean
Mnemiopsis leidyi was
first recorded during late spring-summer 1990 in the Aegean Sea, when it was the most
abundant in gulfs and bays ( Saronikos Gulf (45-75 m-2). In subsequent years M.leidyi
was found in a few numbers in several coastal areas of the Aegean Sea These specimens M.
leidyi may have been carried to the northern Aegean Sea by the Black Sea currents.
The size of individuals collected in the
Aegean Sea and in the Saronikos Gulf was 2.5–6.5 cm(Shiganova et al, 2001).
Further east, M. leidyi appeared
in Mersin Bay in spring 1992 (Kideys & Niermann, 1994), and in Syrian coastal waters
in October 1993 (Shiganova, 1997). It is even less likely that these specimens were
brought with ballast water of the ships as supported by the fact that they were found near
Mersin and Latakia ports.
Effect on the Black Sea ecosystem
The main food of M. leidyi is
zooplankton, but fish eggs and larvae (“ichtyoplankton”) directly follow this.
The fluctuations between ctenophores and
zooplankton were antagonistic (Fig.2). In years with high M. leidyi density,
zooplankton biomass had strongly declined by autumn. When M. leidyi density was
lower, zooplankton biomass remained higher (Fig. 2). During low ctenophore years,
such as 1992-1993 and after 1996, zooplankton, and particularly C. euxinus, showed
signs of a recovery. Copepod species diversity increased; some species, which had
disappeared in 1990-1992, reappeared. The most striking comeback was that of Paracalanus
parvus.
There are three predominantly
zooplanktivorous fish species in the Black Sea: the Black Sea anchovy (Engraulis
encrasicolus ponticus Aleksandrov), Mediterranean horse mackerel (Trachurus
mediterraneus ponticus Aleev) and sprat (Sprattus sprattus phalericus Risso,
1827). They became the main commercial species in the 1980s, after the demise of the large
pelagic piscivorous fish and dolphins . But their stocks and catches declined dramatically
during the bloom of M. leidyi (Fig. 4). The most severe decline was in Black
Sea anchovy and Mediterranean horse mackerel, which spawn during summer and suffered from
decreased zooplankton abundance. Copepoda had been the main food of Black Sea anchovy, but
in 1989 these sharply decreased. Copepods in the diet were replaced for about 30% by
larvae of Cirripedia, Ostracoda and Bivalvia, all with a low caloric content.(Fig.5)
Consequently, growth rate, weight-at-age, fecundity and frequency of spawning of anchovy
decreased (Shiganova & Bulgakova, 2000), while the Mediterranean horse mackerel
completely disappeared from Russian commercial catches.
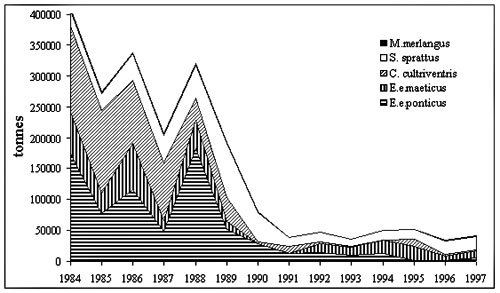
Figure 4. Former USSR
catches of zooplanktivorous fish in the Black and Azov Seas
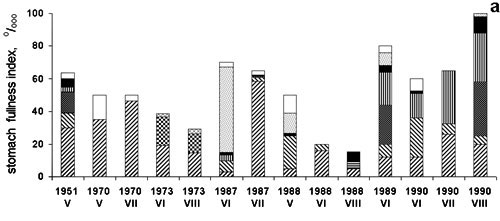
Figure 5. TheBlack
Sea anchovy stomach fullness
There was also an adverse effect on
moderate cold water fish (e.g. sprat), in spite of their spawning in autumn and winter.
These co-occurred with M. leidyi in the intermediate layer in winter and only
occasionally in summer , but there was effect on the food contents and rations also for
sprat.(Shiganova & Bulgakova, 2000). (Fig.6)
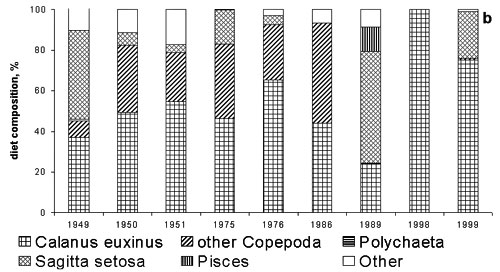
Figure 6. Diet
composition of sprat
M. leidyi affected the food base
of all fish larvae. The percentage of starving larvae increased to high values during the
bloom of M. leidyi. Due to the absence of small copepod species, larvae had to
switch to bigger-sized organisms, which are less suitable and caused a heavy mortality
Effect on the the Azov Sea ecosystem was
even stronger than in the Black Sea. During the first months of summer M.leidyi
consumed almost all of the zooplankton. The stocks of planktivorous fish dropped and
recovered little, due to a persistent summer abundance of M. leidyi .
The strong effect of M.leidyi on
the Black and Azov Seas also reflects an absence of predators. Black Sea mesozooplankton
was decimated by M. leidyi. Planktivorous among fish suffered from competition and
consumption of their pelagic eggs and larvae enormous numbers of M. leidyi,
unchecked by predators of their own. After the appearance of B .ovata, the
situation took a new, spectacular turn: M.leidyi plummeted, and the ecosystem began
to recover (Shiganova et al. 2000). However during seasonal absence of B.ovata
during spring –summer M.leidyi can reach high density and grazes zooplankton to
the level before M.leidyi appearance (Fig.7).
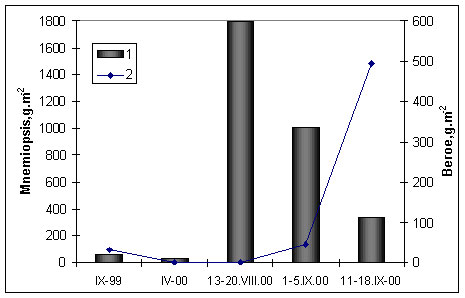
Figure 7.
Biomass of Mnemiopsis leidyi(1) and Beroe ovata (2) during 1999-2000 in the north-eastern
Black Sea
Literature
Kideys, A. E. & U. Niermann 1994.
Occurrence of Mnemiopsis along the Turkish coast. ICES J. mar. Sci.51: 423-427.
Shiganova, T. A.,1993. Ctenophore Mnemiopsis
leidyi and ichthyoplankton in the Sea of Marmara in October of 1992. Oceanology 33:
900-903.
Shiganova, T. A., 1997. Mnemiopsis
leidyi abundance in the Black Sea and its impact on the pelagic community.
In”Sensivity of North Sea, Baltic Sea and Black Sea to antropogenic and climatic
changes” . In E.Ozsoy & A.Mikaelyan (eds), Kluwer Academic Publishers, Dordrecht
/Boston/ London: 117-130.
Shiganova, T. A, 1998. Invasion of
the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic
community structure. Fish. Oceanogr. 7 –GLOBEC Special Issue Ed.Steeve Coombs:305-310
Shiganova, T. A. & Y. V. Bulgakova,
2000. Effect of gelatinous plankton on the Black and Azov Sea fish and their food
resources. ICES J. mar. Sci. 57: 641-648.
Shiganova, T. A, J. V. Bulgakova, P. Y.
Sorokin & Y. F. Lukashev, 2000. Investigation of a new invader, Beroe ovata in
the Black Sea. Biology Bulletin 27: 247-255.
Shiganova T. A., Mirzoyan Z. A.,
Studenikina E. A., Volovik S. P., Siokou-Frangou I., Zervoudaki S., Christou E. D.,
Skirta A. Y., and Dumont H. J.Development population of the invader ctenophore Mnemiopsis
leidyi ( A.Agassiz) in the Black Sea and in other seas of the Mediterranean basin//
Marine biology. 2001. In press.
Vinogradov, M. E., E. A Shushkina., E. I
Musaeva., P. Y. Sorokin,1989. Ctenophore Mnemiopsis leidyi (A.Agassiz) (Ctenophora:
Lobata) - new settler in the Black Sea. Oceanology 29:.293-298.
Vinogradov, M. E., V. V Sapozhnikov &
E. A. Shushkina 1992. The Black Sea ecosystem. Moscow. Russia. Nauka, 112 p. (in Russian).
Volovik, S. P., I. A. Mirzoyan & G.
S. Volovik, 1993. Mnemiopsis leidyi: biology, population dynamics, impact to the
ecosystem and fisheries. ICES. (Biol. Oceanogr. Committee) 69: 1-11.
|
