Ahmet E.
KIDEYS
Institute of Marine Sciences, Erdemli, Turkey (kideys@ims.metu.edu.tr,)
Galina A. FINENKO
Institute of Biology of the Southern Seas, Sevastopol, Ukraine (shulman@ibss.iuf.net)
Alona ARASHKEVITCH, Tamara A.
SHIGANOVA
P.P. Shirshov Institute of Oceanology RAS, Moscow, Russia (shiganov@sio.rssi.ru),
Abolghasem ROOHI, Mojgan ROUSHAN
TABARI, Mohammad RUSTEMIAN, Arash GEVANSHIR
Mazandaran Fisheries Research Center, Sari, Iran (roohi_ark@yahoo.com)
AliRiza MIRZAGANI, Siamak BAGHERI
Guilan Fisheries Research Center, Anzali, Iran (roohi_ark@yahoo.com)
Hossein NEGARESTAN, Farokh
PARAFKANDEH
Iranian Fisheries Research Organization, Tehran, Iran
SUMMARY
A team of scientists from Turkey, Ukraine
and Russia transported the ctenophore Beroe ovata from the Marmara Sea to the
Mazandaran Fisheries Research Station (Sari, Iran) on the southern Caspian coast. The
objective of the study was to investigate propagation of this species in Caspian Sea water
in the laboratory. The experimental period was between 25th September and 4th
October 2002. The results of experiments were unsuccessful since spawning and hatching
rates were very low and, none of the larvae developed into adult stage. Based on our
independent recent experiments in the Black Sea just prior to those in the Caspian, we
believe that the main reason for our failure was the delay in performing experiments with B.
ovata. Detailed results of experiments are given. More importantly, based on our
previous and these experiments, comments for the success of a similar experiment for the
next year along with points needed to be taken into account for the introduction are
provided.
INTRODUCTION
Invasion of the Caspian Sea by the Mnemiopsis
leidyi has become a major environmental issue threatening the fragile ecosystem of
this globally unique water-body. M. leidyi has now reached critical biomass levels
in the most of important commercial areas of the Caspian Sea and is jeopardizing the
fishery industries through a serious impact on the food chain in general and on pelagic
fish in particular. The same species had caused a similar impact on the ecosystem of the
Black Sea from where it was transported into the Caspian Sea.
The appearance of a new ctenophore, Beroe
ovata, which feeds almost exclusively on M. leidyi has started a new era since
the late 1990s in the Black Sea. B. ovata reversed the disastrous situation caused
by M. leidyi in the Black Sea, reducing the latter to low levels as well as
limiting its appearance to a narrow period throughout the year. Thus with the decimation
of M. leidyi levels, zooplankton production and hence fish recruitment has been
restored. No species appear to have been lost from the Black Sea fauna during the recovery
process even if, at the height of the M. leidyi outbreak, many fell to levels so
low as to make their observation impossible.
It should be stressed that B. ovata
is likely not a miracle solution: it is a thermophilic species, less tolerant of low
salinity than M. leidyi. However it seems a perfect biological control agent
against M. leidyi. Even, as a free-living larva, it relies on M. leidyi
(i.e. on its larva but also on the tissue of larger specimens). Inter alia, this
makes it unlikely that B. ovata will ever be able to turn to sources of food other
than M. leidyi in the Caspian. It is also a voracious feeder, meaning that it needs
lots of prey to survive and thrive. As a result, it only peaks after the abundance peak of
its prey, which it rapidly reduces to low numbers, but thereafter it collapses itself. It
is highly unlikely that such a predator will eliminate its prey completely, and therefore,
we will have to live with both jelly species in the Caspian environment, albeit at
tolerable levels.
Because of the adverse impact of M.
leidyi, the region has discussed and agreed to form a Regional M. leidyi
Advisory Group. This group has developed M. leidyi Control Strategy and has carried
out a number of monitoring and research activities. Based on the Strategy, deliberations
of the M. leidyi Advisory Group and the findings of the activities the region has
opted to take biological measures to deal with the M. leidyi issue. More
specifically the Advisory Group has agreed to start studies to introduce B. ovata
as a predator for M. leidyi after being convinced of the soundness of the likely
environmental impacts of such intended introduction.
In framework of this program, special
experimental studies were performed in Mazandaran Fisheries Research Center of Iran in
2001. The results of these studies confirmed that B. ovata could live and grow in
the Caspian Sea water with a salinity of around 12 ppt (see Kideys et al. 2001). However
the reproduction was very low and only a few larvae could hatch. These larvae died before
developing into adult stage. For the success of introduction, it is desirable to be able
to propagate the B. ovata in captivity. Production of early stages of B. ovata
can provide the seeding stock in a future release program, after necessary prerequisites
are met. A team of scientists has been performing miscellaneous experiments with B.
ovata in the northern (Dr T. Shiganova) and southern Black Sea (Drs A.E. Kideys, A.
Arashkevitch, G. Finenko) during the early September 2002. This team has flown to
Mazandaran to continue experiments with Caspian water using local M. leidyi as food
source (Photo 1) during 25 September - 5th October 2002. Thus, the main aim of
our mission in Iran was to propagate B. ovata. In the last days of the mission, a
mesocosm experiment was also started with the surviving B. ovata.

Photo 1. The research team performing B. ovata propagation experiments during
Sep-Oct 2002 in Mazandaran, Iran
MATERIAL AND METHODS
B. ovata were collected from the
northern Marmara in Istanbul, Turkey (salinity 22 ppt). B. ovata individuals
collected were of 10-50 mm length and transported in two batches. For the first batch, 47
individuals of B. ovata were sampled during 23-24 September 2002. In the
second batch, 28 specimens were collected from the same area next day (26 September
2002). The two batches were transported separately by plane to Tehran and driven to the
laboratory in Khazerabad (Mazandaran, Iran) in two 5 l jars.
The first batch of B. ovata was used
in experiments for acclimation to lower salinity. All 28 individuals from the second batch
were kept in Marmara water (salinity 22ppt) as control for fecundity and larval
development to compare with those in the Caspian Sea water.
In the acclimation experiments (for the all
47 individuals), B. ovata were kept (details given in Table 1) in 3 l containers at
an initial density of 7-15 individuals. B. ovata were fed on M. leidyi, and
ingestion and mortality were recorded daily. After acclimation, B. ovata were
transferred into the Caspian Sea water (12.5) aquaria individually, larger ones (>15
mm) in 3-liter aquaria, and smaller individuals in 1 liter aquaria. Transfer to lower
salinity, water change and feeding were done daily.
Table 1. Acclimation of B. ovata to
Caspian water salinity in 2002.
| Venue, Number of B.
ovata |
Date |
Time |
Salinity (PTT) |
| Marmara |
23 - 24 9/2002 |
02:00 |
22 |
| Mazandaran |
26/9/2002 |
16:00 |
21 |
| 47 |
27/9/2002 |
10:00 |
20 |
| 40 |
27/9/2002 |
17:00 |
18 |
| 35 |
28/9/2002 |
10:00 |
16 |
| 35 |
29/9/2002 |
11:00 |
12.9 |
| 35 |
29/9/2002 |
16:00 |
12.5 (Caspian water) |
Containers with
Caspian Sea water were provided with aeration. After B. ovata acclimation to the
Caspian Sea water salinity, the experiments on survival, feeding and reproduction rates
were started.
Control experiments in Marmara Sea water
were performed in a total of 6 containers. Two of them had a capacity of 5 liters having
13 B. ovata individuals of 14- 39 mm length. Other four control containers with a 2
liters capacity had one individual each (Table 2). During experiments all individuals were
fed and checked for ingestion and reproduction daily.
The room temperature was kept at 240.
Since only a 20 liter filtered seawater was brought, the filtered (through 50 um mesh)
Marmara seawater was reused.
Towards the end of our propagation
experiments (30 Sep 2002), surviving B. ovata individuals were used to initiate a
preliminary mesocosm experiment in the Caspian water. For this 6 large tanks (being three
controls and three experimental tanks) of 5 tons capacity were filled with Caspian water
which included zooplankton and M. leidyi. In three tanks, 5 B. ovata were
added each, though their conditions were not generally good as they were not actively
feeding and spawning even during the experiments. All tanks would be maintained with
sufficient number of relatively large M. leidyi as food for the B. ovata.
With every three days intervals, 10 lt seawater from each tank would be filtered to
quantify M. leidyi and zooplankton to see the impact of B. ovata on the
mimicked pelagic ecosystem. This experiment will provide a good experience for the main
mesocosm experiments we plan to perform in very large outdoor tanks (10 m in diameter; see
Photo 2) in Mazandaran in 2003 summer.
Table 2. Reproduction and feeding of B.
ovata in Marmara water.
Container No |
Volume
(l) |
# of B. ovata |
Size (mm) |
Duration (days) |
# of prey
consumed |
# of Eggs |
1 |
2 |
1 |
35 |
7 |
7 |
162 |
2 |
2 |
1 |
46 |
7 |
7 |
|
3 |
2 |
1 |
50 |
7 |
7 |
|
4 |
2 |
7 |
22ñ 6 |
7 |
21 |
3 |
5 |
5 |
4 |
36ñ 4 |
7 |
28 |
|
6 |
5 |
9 |
21ñ 5 |
7 |
54 |
54 |

Photo 2. Large (10 m in diameter and 2 m deep) outdoor mesocosm tanks which are being
built in Mazandaran, Iran.
RESULTS
Acclimation of Beroe ovata to the
Caspian Sea water salinity
Acclimation of Marmara Beroe ovata
to the Caspian Sea water salinity was performed by changing salinity step by step from 22
ppt to 12.5 ppt during 3 days (Table1). During acclimation, individuals B. ovata
were offered several individuals of M. leidyi but they did not feed during
acclimation.
Survival of B. ovata in the Caspian
Sea water was estimated in three size groups: 10-19 mm, 20-29 mm, and ? 30 mm (Fig.1).
Survival was highest (91%) in the smallest size group, medium (61%) for the middle size
group, but lowest (33% denoting to only two survivors) among the large ctenophores during
five days of experimental period.
Survival of B. ovata individuals
(total 23) in the Marmara water was 100% in all size group.
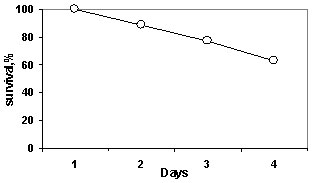
Fig. 1. Survival rate of B. ovata in Caspian Sea water in 2002.
Reproduction
Reproduction was observed in two sets of
experiments, in the Marmara Sea water and the Caspian Sea water. Aquaria with B. ovata
were carefully examined every day in the morning with the aim to check eggs (see Photo 3)
of B. ovata.
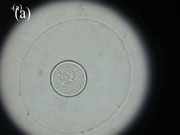 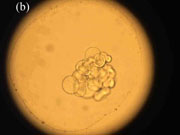 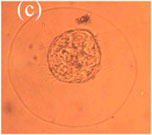
Photo 3. (a) Normal B. ovata egg, (b) Dividing egg, (c) Larva about to hatch
(Photographs by A.E. Kideys)
Reproduction in the Marmara Seawater
All B. ovata kept in the Marmara
Seawater were in good conditions. All of them were active and consumed M. leidyi
intensively every day.
Nevertheless their reproduction rate was
very low if any (Table 2) Only in three cases we observed egg production. B. ovata
of 35 mm length produced a total of 162 eggs (Container 1) during the entire experimental
period. In another aquarium having nine small individuals of B. ovata, a total of
54 eggs were collected. In the third container, there were a total of three eggs. Most of
the collected eggs (more than 90%) did not develop; probably because they were not
fertilized (Fig. 2).
Reproduction in the Caspian Sea
waters
None of B. ovata kept in the Caspian
Sea water seemed active; they tended to be near the bottom and almost did not consume any
food. But we observed spawning in four containers, moreover, eggs were developed and
hatched (Fig. 2, Photo 3). However they did not grow.
Table 3. Reproduction and feeding of B.
ovata in the Caspian Sea water.
Container
No |
Volume
(l) |
# of B.
ovata |
Size, (mm) |
Duration (days) |
#
of prey consumed |
# of eggs |
1 |
3 |
1 |
30 |
5 |
|
|
2 |
3 |
1 |
35 |
1 |
|
|
3 |
3 |
1 |
48 |
2 |
|
|
4 |
3 |
1 |
28 |
2 |
|
|
5 |
3 |
1 |
40 |
5 |
|
|
6 |
3 |
1 |
30 |
3 |
|
|
7 |
3 |
1 |
30 |
4 |
|
|
8 |
3 |
1 |
28 |
3 |
|
1 |
9 |
3 |
1 |
29 |
3 |
|
|
10 |
3 |
1 |
28 |
5 |
|
12 |
11 |
3 |
1 |
25 |
5 |
|
|
12 |
3 |
1 |
20 |
4 |
|
1 |
13 |
3 |
1 |
25 |
4 |
|
|
14 |
3 |
1 |
28 |
5 |
|
|
15 |
1 |
1 |
12 |
5 |
2 |
|
16 |
1 |
1 |
23 |
5 |
|
|
17 |
1 |
1 |
17 |
5 |
1 |
|
18 |
1 |
1 |
15 |
1 |
|
|
19 |
1 |
1 |
20 |
4 |
|
|
20 |
1 |
1 |
25 |
5 |
1 |
2 |
21 |
3 |
1 |
24 |
5 |
|
1 |
22 |
3 |
1 |
20 |
5 |
|
|
23 |
1 |
2 |
15,25 |
5 |
|
|
24 |
2 |
1 |
11 |
5 |
|
|
25 |
1 |
2 |
10,10 |
5 |
|
|
26 |
1 |
1 |
11 |
5 |
|
|
27 |
1 |
1 |
12 |
5 |
|
|
28 |
1 |
1 |
11 |
5 |
|
|
29 |
1 |
1 |
12 |
5 |
|
|
30 |
1 |
1 |
18 |
5 |
|
|
31 |
3 |
1 |
17 |
5 |
|
|
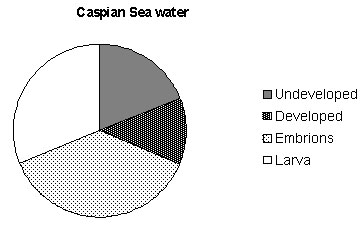
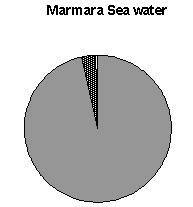
Fig. 2. Hatching success of Beroe ovata eggs in the
Caspian Sea and Marmara Sea waters.
Mesocosm experiment
The results of this preliminary experiment
were not analysed, yet. However, since the condition of ctenophores were not good at the
start of experiment, most were dead within a few days. However, a few seemed actively
feeding on M. leidyi. One specimen was alive at the end of planned one-month
experiment.
CONCLUSIONS
Overall we failed in our task (i.e. mass
spawning and growing larvae into adults). We believe that the main reason for this failure
is our late travel to Iran to perform experiments. During our similar experiments last
year in Mazandaran, which had started on 11th September 2001, we had obtained a
total of 138 eggs of B. ovata from which 7 larvae had been hatched. This is much
higher number compared to 20 eggs obtained from recent experiments in Caspian Sea water.
Our independent experiments in the northern and southern Black Sea show that average egg
numbers decrease with the time for the freshly collected B. ovata specimens.
Therefore we think that their peak spawning period is as narrow as two weeks during the
course of the year starting in August ending by the early September depending on the
population dynamics of the prey (i.e. M. leidyi).
Comments for next year B. ovata
transfer
It is thus clear that timing in B. ovata
spawning is very critical. Therefore, any activity regarding propagation should be
undertaken within two weeks of high spawning capacity of the ctenophore being between the
second half of August and early September.
Collecting B. ovata from the Black
Sea rather than Marmara would save time of acclimation. Searching for less saline areas in
the Black Sea as the source region of B. ovata could be very useful.
B. ovata adults are sensitive to
handling (and larvae are even more sensitive), and therefore their transport via tanker
etc does not seem practical, making the air the only route, if it comes to that. For the
next year planned introduction activity, perhaps use of helicopters could be even better
if the collected B. ovata could be transported directly to Sari or other regions
for release after acclimation. Laboratory experiments performed in the northern and
southern Black Sea by our team show that egg production is highest during the first 24
hours following the sampling and spawning rate decreases dramatically with every other
day. Therefore introducing B. ovata into the Caspian Sea water as early as possible
following the sampling from the Black Sea is important for a fruitful introduction
program. In addition to adults, transport of larvae could also be considered as they are
easier to adapt to lower salinity, provided handling is minimal.
Necessary custom-related permissions should
be completed before such transport. Adequate quantities of Black Sea water of should be
transported with a truck tanker to Mazandaran to be used for acclimation purposes for the
transported individuals, before transporting the B. ovata.
Unfortunately since we failed to reproduce
them in desirable quantities, we should still consider bringing high number of B. ovata
for introduction purposes (see "PROPOSAL FOR PERFORMING STUDIES ON THE PROPAGATION OF
CTENOPHORE BEROE IN THE CASPIAN WATER" submitted to CEP in 2002).
Because of presence of large outdoor tanks,
main activities are planned to be undertaken in Mazandaran. However, a potential
introduction will be at several points as was mentioned in the report mentioned above. We
should also investigate regions with high salinity in the southern Caspian Sea among
potential release sites.
The last but not the least, necessary
funding should be secured for completing the necessary studies (i.e. those relating
mesocosm and parasite) and to be ready for the introduction.
Acknowledgements
This study was made possible by
organizational efforts of DrsTimothy Turner, Hamit Gaffarzadeh and Vladymyr Vladymyrov of
Caspian Environment Program (CEP) under financial support of UNOPS.. We greatly appreciate
kind help of Mazandaran Fisheries Research Station staff (particularly Dr Hosseinali
Rostami, Mr A. Salmani, Mr M. Najafpour and Mr Zekeriya Yahyai) in sampling and help
during our laboratory experiments, and to Iranian Fisheries Research Organization (IFRO)
in Tehran for logistics. |
