|
|
|
|
 |
The Black Sea has a remarkable chemistry that distinguishes it from every other sea on our planet. What makes it really special is that whereas all other seas and oceans (with a few isolated exceptions) have oxygen dissolved in their waters, there is absolutely no oxygen in the Black Sea below a depth of about 100 metres (Figure 1). Furthermore, the anoxic (oxygen free) water has a large concentration of hydrogen sulphide, a very poisonous gas that smells of rotten eggs. With the exception of a few highly specialised life forms, most animals and plants cannot survive in anoxic conditions. Hydrogen sulphide is also very poisonous to humans. The surface waters of the sea are less dense than the deeper anoxic layers because they are warmer and have a lower salt content. Fortunately for life in and around the Black Sea , this density difference prevents the anoxic bottom waters from ever reaching the sea surface. |
| |
|
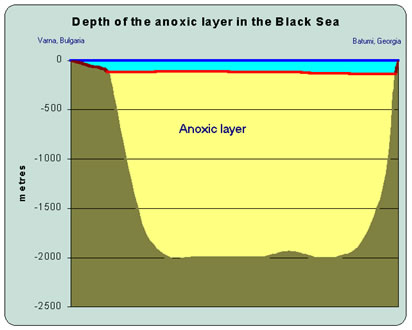 |
Figure 1. A West-East section of the Black Sea from Varna to Batumi showing the huge volume of anoxic water. Note the shallow shelf off Varna with oxygenated water down to the sea floor |
How did this situation develop? The deep waters of the Black Sea consist of water that has travelled from the Mediterranean through the Sea of Marmara and the Bosphorus Straits (also known as the Turkish Straits). The Bosphorus too has two layers with outflowing Black Sea water at the top and Mediterranean water at the bottom. The Bosphorus is only about 50m deep (even shallower in places) and 700m wide and the incoming Mediterranean water spills into the deepest part of the Black Sea nearly 2Km below. For the bottom layer of the Black Sea – almost 95% of the Sea's volume – this incoming water is the only source of oxygen and the new water may stay as much as 1000 years in the bottom before eventually mixing with enough fresh water to reach the surface and start its journey back to the Mediterranean .
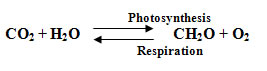
Plants use the sun's energy to convert carbon dioxide to living matter and oxygen, a process known as photosynthesis. At night the reverse process, respiration, occurs and some of the oxygen and living matter are consumed and energy is released. The same processes apply to the tiny free floating plants – phytoplankton – in the sea or the algae found nearer the shore. The photosynthesis equation, without which there would be no oxygen in our atmosphere is written like this (note that CH2O is used to represent carbohydrates, the most abundant building block for living beings):
| (1) |
 |
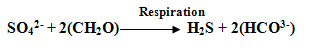
The sub-surface layers of the sea receive a constant shower from above of dead plants and animals and their excrement and these are subjected to bacterial decay, also consuming oxygen. In the case of the lower layer of the Black Sea , the oxygen demand from organic debris is much greater than the supply of water rich in oxygen from the Mediterranean . This explains why the lower layer of the Black Sea is anoxic. The hydrogen sulphide in this layer is produced by bacteria that find an alternative supply of oxygen locked up in the sulphate ions that are relatively abundant in seawater:
| (2) |
 |
There are many fascinating processes going on in the deep Black Sea . The environment is thought to resemble that of the early oceans, before there was oxygen on our atmosphere and this fact attracts considerable scientific research. The bottom sediments of the Black Sea for example contain rich deposits of methane that has solidified like ice (methane hydrates) at the low temperatures and high pressures of the sea floor. The methane was produced as a final product of the degradation of thousands of years of accumulated organic matter and may provide a future fuel supply. Even this does not remain intact however. Recently discovered primitive micro-organisms, the archaeans, can slowly oxidise the methane to carbonates forming a crust or chimneys above the sea floor. In some places methane gas is bubbling from the chimneys but this rarely reaches the sea surface as it is dissolved en-route. Stories of the Black Sea ‘catching fire' refer to the methane but are unlikely to be true as most of the gas bubbles exchange with dissolved nitrogen gas during ascent through the water column. Most of what emerges would not be flammable. Only a methane seep in shallow water could conceivably be flammable and reports of methane seeps in the Black Sea are usually from water of at least 100m depth. |
|
| Nitrogen and Phosphorus loads to the Black Sea determine its fertility and have led to eutrophication. |
|
The photosynthesis equation (1) does not fully describe the production of algae and plants in the Black Sea . Plants need other substances in order to grow. These include ‘nutrients', compounds of nitrogen and phosphorus and in some cases silica as well as a range of minerals such as iron, manganese and vanadium and organic stimulants such as vitamins. Growth of phytoplankton (the tiny floating plants that are the basis of ocean food chains) may be limited by the supply of nitrogen, phosphorus, silica and iron.
Much of the nutrient supply to marine ‘primary producers' (a collective term for photosynthetic phytoplankton, nearshore seagrasses and algae) in the surface waters of the sea comes from recycling. During the process of respiration and bacterial decay, nutrients are released back into the water column and can be taken up by new generations of primary producers. Some nutrients are lost however, flushed out of the system, permanently buried in the sediments or even removed as fish for human consumption. The loss is compensated by the arrival of new nutrients from rivers, the atmosphere and deep waters. This is where the problem starts for the Black Sea . Human activities on land have increased the flow of nutrients to the sea leading to an enormous boost in primary production; too much for the system to cope with and placing an even larger demand on the limited oxygen supply to break down the excess organic matter. This phenomenon, known as eutrophication, caused serious degradation of the Black Sea ecosystem, particularly its North-West Shelf, since the 1970s. It resulted in seasonal hypoxia (very low levels of oxygen) and occasional anoxia on the previously well-oxygenated NW Shelf.
f the limiting nutrients, nitrogen has the most complex chemistry. In well-oxygenated waters, the nitrogen in natural organic matter is eventually oxidised to nitrate (NO32-) and this is the most abundant nitrogen compound in the sea. The less oxidised form, nitrite (NO2?), is also present however, particularly where oxygen concentrations are low. The reduced form of combined nitrogen, ammonia, is exuded by many marine organisms and is the most stable form in anoxic waters such as those found in the lower layer of the Black Sea . It exists as an equilibrium between the gaseous and ionic forms:
| (3) |
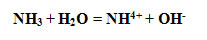 |
Dissolved nitrogen gas is abundant in the sea but very few marine organisms are capable of using it as a nutrient source. The ‘nitrogen fixation' process requires a large amount of solar energy and dissolved iron; both are in limited supply in the Black Sea .
The human activities causing eutrophication are mostly related to the way we produce our food and dispose our sewage. Annual fertiliser use in the Danube basin rose from 1.3 million tons per year in 1961 to 4.8 million tons in 1981 and an increasing proportion of the population was connected to sewerage (but not to sewage treatment plants!). Intensive agricultural activities made the problem even worse. The nutrient runoff to the Danube has declined again in recent years because of the economic costs to farmers of applying large amounts of fertilisers and the decline of large collective farms. There is concern that it will rise again as economies become stronger but better farming practices can help to keep excess nutrients where they belong, on the land.
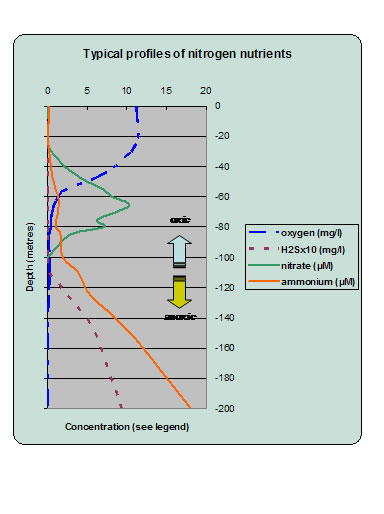 |
Fig 2. A typical profile of oxygen, hydrogen sulphide, nitrate and ammonium from the western Black Sea . Surface nitrate is low because it is consumed by phytoplankton (data from Yakubenko and Lukashev, cited by Sorokin, 2002) |
|
| Chemical (including oil) pollution is not as bad as we first imagined but risks of serious incidents are increasing. |
|
Pollution occurs when chemicals or energy (noise or heat) released into the environment cause damage to any form of life or to the value of the environment to humans (for amenities or other uses). There is plenty of evidence that many rivers discharging to the Black Sea were seriously polluted in the decades of the 70s and 80s. Some of the sources of pollution, heavy industries and agrochemicals such as pesticides, have now declined due to the economic collapse that affected many countries in the 1990s and to a lesser extent due to better industrial processes and waste treatment. Oil shipments through the Black Sea have risen sharply however and there is a constant risk of oil spills through accidents or operational discharges (leaky pipes, careless handling, and illegal washing of ships' tanks). Pollution can be prevented in all cases, it is the result of accidents and the intentional use of the environment as a means to dispose of waste.
The main classes of potential pollutants are shown on Table 1:
Table 1. Potential pollutants to the Black Sea
Pollutant class |
Source |
Situation in the Black Sea |
Synthetic organic chemicals
Several thousand chemicals including very toxic substances such as:
• Pesticides
• Solvents
• Dioxins (from burning plastics)
• Polyaromatic hydrocarbons (PAHs)
|
Agriculture:
Pesticides are sometime applied in large quantities to kill unwanted insects or to remove weeds or fungus infections. Some of these compounds are very persistent and toxic.
Industry:
Industrial process use and produce thousands of different chemicals. Without proper care, these can be released to the environment as waste during production or later on when the product is used somewhere else
Home:
We use large amounts of chemicals at home. Some plastics for example, will release toxic dioxins when they are burned – avoiding excessive waste and its proper disposal is very important.
|
It is very difficult, time-consuming and expensive to measure organic chemicals that may cause pollution. Our knowledge of their concentration and distribution in the Black Sea is poor. We do know that pesticide concentrations are currently lower than most other European Seas because of the economic difficulties for farmers to purchase them. |
Oil
Crude oil contains thousands of different hydrocarbons. These are compounds of carbon and hydrogen that may be saturated (contain no double bonds), unsaturated (contain double or triple bonds and therefore more reactive) or aromatic (contain benzene rings)
In general terms, the larger the number of benzene rings or double or triple bonds in an oil component, the more toxic it is likely to be. |
Maritime transport :
Most of the oil entering the sea from ships does not come from large oil spills but smaller more frequent discharges through emptying ballast water, cleaning tanks and bilges (bilges are the bottom part of a ship where any water or other spilt liquids will gather) and broken connections when loading and unloading. Of course, large spills produce spectacular local damage.
Production and refining
Oil based lubrication is sometimes still used when drilling oil and gas wells. This causes a major local impact.
Refineries occasionally discharge oil products but sufficient technology exists to prevent this from occurring.
Urban sewage
This is the biggest single source of oil to the sea. Careless disposal of car engine oil down the drain is a major factor but there may be contributions from public transport and industry .
|
Though shipments of oil have increased significantly it is fortunate that there have been few major oil spills in the Black Sea . However, oil is frequently found on bathing beaches and the concentration of dissolved oil is higher than the neighbouring Mediterranean . This appears to be the result of two factors:
A large number of operational discharges from maritime transport (ballast tank discharges are thought to be a major problem)
Urban sewage and industrial waste discharged to rivers and the sea. Very large amounts of oil enter the sea from its tributary rivers but the same is occurring in coastal towns and cities.
For more information see the study sheet on shipping |
| |
|
|
Heavy metals
Heavy metal is a term loosely used to describe transition elements and their compounds (such as lead, copper, cadmium, mercury, tin, zinc, etc.)
Heavy metal pollution is a major problem in fresh waters (serious health consequences for humans drinking contaminated water) but is less widespread in the marine environment. There have been serious local problems with mercury pollution however and the use of the organotin compound tributyl tin (TBT) is severely restricted owing to its effects on marine life.
|
Mining
Mining operations and the disposal of mine waste is a major source of heavy metals. Waste is sometimes stored in open ponds that can overflow into rivers.
Industry
The metallurgical industry is a source of heavy metals but many other industries use metal compounds and produce waste. The leather processing (tanning) industry for example uses large amounts of toxic chromium for example.
Transport
In order to prevent marine organisms from growing on ship's hulls, antifouling paints are often employed and these traditionally contained copper. TBT was found to be more effective but has serious consequences for the marine environment.
Energy
Batteries often contain large amounts of heavy metals, typically lead or cadmium. Proper disposal is essential but they are commonly included in household or industrial waste. |
There is little evidence of general serious heavy metal pollution in the Black Sea but there are some local areas of local contamination associated with industry or mining.
Accidents such as that of the Baia Mare mine in the Danube basin (massive spillage from a gold mine waste pond that killed all animals in over 200 Kms of the river Tisa ) are a risk in many Black Sea countries |
Radionuclides
These are elements that emit radioactivity. They can be naturally occurring lathanides and actinides or human produced transuranic elements. Elements such as uranium occur in nature and a certain ‘background' level of radioactivity is always present.
|
Mining
The production of nuclear fuel (for reactors, medical and industrial use or weapons) requires the processing of very large amounts of uranium ore. This releases large amounts of radioactive waste to the environment.
Nuclear energy
Normally emissions from nuclear reactors are very tightly controlled. There is an accident risk however and long-term storage of spent fuel is difficult and costly.
Other activities
Medical, military and industrial uses may produce some contamination but this is limited compared to other sources
|
The Chernobyl nuclear accident in 1986 caused immense human suffering and environmental contamination, particularly on parts of the Dnieper river that drains to the Black Sea . The dams in the river prevented most of the pollution from reaching the Black Sea however. Because of this accident and other operational discharges, the Black Sea has approximately double the radioactivity than the Mediterranean but these levels are well below those that would cause significant damage to humans and animals |
Solid waste
The presence of garbage in the sea is a major problem as it damages amenities and habitats. Plastic waste is a particularly difficult problem. |
Urban and ship waste disposal
Garbage mostly comes from packaging materials that are disposed of by householders or industry |
Many Black Sea beaches are seriously littered with garbage. This comes from poor urban disposal, improper disposal of ship's waste and careless citizens. There is little information on garbage at the bottom of the sea. |
|
|

