Predatory bird health - white-tailed sea eagle

|
||||
Key message
Strong relationships have been found between white-tailed sea eagle reproductive ability and concentrations of DDTs and PCBs in their eggs (12). Reproduction in the Baltic eagle population in the 1970s was reduced to 1/5 of the pre-1950 background level. Following bans of DDT and PCB during the 1970s around the Baltic, eagle productivity began to recover in the 1980s and since the mid-1990s is largely back to pre-1950 levels. The population on the Swedish Baltic coast has increased at 7.8 % per year since 1990.
On the Swedish coast of the Bothnian Sea, nestling brood size remains below the pre-1950 level and the occurrence of dead eggs is significantly higher than in the Baltic Proper and Bothnian Bay, indicating a possible impact from other contaminants (10).
This fact sheet includes data from two coastal populations in Sweden and from a sample of coastal and inland populations in Germany (Federal State Mecklenburg-Western Pomerania).
Results and assessments
Relevance of the indicator for describing developments in the environment
The white-tailed sea eagle was the first species that signalled about deleterious effects from environmental pollutants in the Baltic Sea. If white-tailed sea eagle reproduction had been monitored earlier during the 20th century, the negative impact of DDT could have been signalled as early as in the 1950s in the Baltic Sea. The sea eagle is the ultimate top predator of the Baltic ecosystem, feeding on fish, sea birds as well as on seals, and is thus strongly exposed to persistent chemicals that magnify in the food web.
Eagles are presently breeding along the coasts of the whole Baltic Sea, and are monitored in a network of national projects with harmonized methodology. Monitoring of sea eagle reproduction in Sweden is included in the National Environment Monitoring Programme since 1989 as indicator of effects from chemical pollutants. Pre-1954 background data on breeding success and pre-1950 background data on nestling brood size are available as reference levels for evaluation of observations under the programme (5, 7).
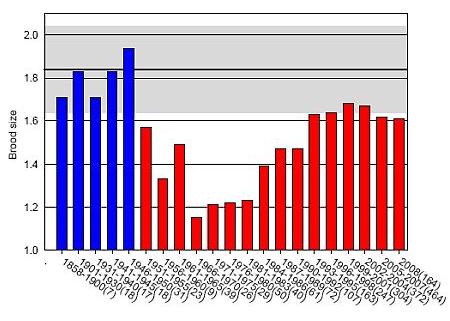
Figure 1. Mean brood size of white-tailed sea eagle on the Swedish Baltic coast over time. Sample size for each time period is given in brackets. Reference level based on 1858-1950 is given with 95 % confidence limits according to (7).
Policy relevance and policy references
The improvement in reproduction of the Baltic white-tailed sea eagle populations came no earlier than 10 years after most countries around the Baltic had implemented bans of DDT and PCB. This is a clear reminder of the potentially long-term effects from persistent pollutants. The subsequent recovery, from an 80 % reduction in reproductive ability in the 1970s, is nevertheless an important evidence of successful results from wise political decisions.
The maintenance of viable populations of species is one important objective of the HELCOM Baltic Sea Action Plan. EU Birds Directive (79/409/EEC) lists the white-tailed sea eagle in Annex I, binding member states to undertake measures to secure reproduction and survival of the species. The species is listed in the following international conventions: Bern Convention Annex II (strictly protected species), Bonn Convention Annex I and II (conservation of migratory species), Washington Convention (CITES) Annex I (regulating trade). The maintenance of healthy populations of white-tailed sea eagle is in accordance with the following four Environmental Quality Objectives defined for Sweden:
- A non-toxic environment;
- A Balanced Marine Environment;
- Flourishing Coastal Areas and Archipelagos;
- A Rich Diversity of Plant and Animal Life.
Monitoring of sea eagle population health as environmental indicator, as well as monitoring of contaminants in eagles and their prey, is recommended in an international Species Action Plan, adopted under the Bern Convention in 2002 (14).
Conservation targets
The reproductive parameters of white-tailed sea eagles should not be influenced by chemical contaminants. The breeding success should be > 60 %, the productivity >1.0 nestling/checked territorial pair (both values measured as average of the last 5 years). These thresholds are based on the lower ends of the 95% confidence limits for estimated background brood size and breeding success on the Swedish Baltic coast (7) and thus refer to the coastal populations. (Note: It has not yet been validated whether these threshold values are true for all coastal regions of the Baltic Sea. Future regional specification may be necessary).
Assessment
White-tailed sea eagle population health status is assessed by monitoring the following indicators:
Reproduction
Contaminant burdens
Nutritional condition of nestlings
Death causes of specimens
Reproduction
White-tailed sea eagle reproductive ability is monitored annually by assessing the frequency distribution of occupied eagle nests containing [0], [1], [2] and [3] nestlings (3 being the maximum in this species). Survey techniques and sampling methods are presented in (6, 9,11). Three indicators of reproductive ability are calculated from these data:
Breeding success – the proportion of nests containing at least one nestling of at least three weeks of age, out of all occupied nests ([n1] + [n2] + [n3] / [n0] + [n1] + [n2] + [n3]).
Trends in breeding success of sea eagles on the northern, central and southern Baltic coast over time are presented in Figure 2. As the population grew over the study period, the number of annually checked pairs increased: in the Baltic Proper from 20–30 before 1975 to 176 in 2006, and in the Gulf of Bothnia from around 10 before 1975 (all in the Bothnian Sea) to 89 in 2006 (as the eagle had repopulated also the Bothnian Bay). Similarly, the number of annually checked pairs in the sample from Mecklenburg-Western Pomerania, Germany, increased from around 75 to 219 between 1973 and 2008. Retrospective studies have shown that the breeding success on the whole Swedish Baltic coast decreased from on average about 72 % before the 1950s to 47 % in 1954-1963 and 22 % in 1964-1982 (3). Breeding success increased significantly in the Baltic Proper as well as the Gulf of Bothnia from the early 1980s (Figure 2). By the middle to late 1990s, breeding success in both areas was no longer significantly different from the background level. The development in the southern Baltic (Germany) is similar to that in the central Baltic (Sweden, Baltic Proper, see Figure 2), but the breeding success seems to have stabilized at a lower level in Germany. The difference between the German sample and the two Swedish samples, respectively, is statistically significant. Impacts of intraspecific competition in areas with high density of breeding pairs has been discussed as a possible reason for the lower breeding success in Mecklenburg-Western Pomerania (1).
Nestling brood size – the mean number of nestlings of at least three weeks of age in nests containing young ([n1] + [n2x2] + [n3x3] / ([n1] + [n2] + [n3].
Based on data from nests inspected by climbing the nest tree, and excluding nests checked only from the ground, nestling brood size is a precise standard. Nestling brood size began to increase in both areas from the 1980s, roughly in synchrony with the increase in breeding success (Figure 3). This is inherent with an improvement in the hatching success of the eggs, affecting both these indicators in parallel. Brood size reached back to the pre-1950 reference level in the Baltic Proper in the late 1990s. In the Gulf of Bothnia, however, brood size is still significantly below this reference level. This is mainly due to smaller broods in the southern part of the Bothnian Sea, as illustrated in Figure 4.
The current brood size in Germany is lower than in Sweden (Figure 3, 4). During the period 1996-2004, 1.48 nestlings/nest have been recorded in Mecklenburg-Western Pomerania. It should be mentioned that this sample includes data from nests only checked from the ground, which results in a certain error due to nestlings not visible from this position. However, this bias does not explain the full difference from the data obtained for Sweden. Data received from ground observations in Germany underestimated the real number of nestlings by 11%. (2). Using this correction factor for the nests not climbed (about 50 % of the total German sample), the corrected brood size for Mecklenburg-Western Pomerania is 1.56, which is still clearly on the low side compared to most coastal records from Sweden (Figure 4).
Productivity - the mean number of nestlings of at least three weeks of age, out of all occupied nests ([n1] + [n2x2] + [n3x3] / [n0] + [n1] + [n2] + [n3]).
This indicator combines the two indicators above into one and assesses the reproductive output of the population. It is a most useful indicator in studies on relationships between reproduction and contaminants, as illustrated in Figure 5. It is also a vital parameter in assessments of population status in management perspectives.
As nests are climbed for assessment of the reproductive parameters, nestlings are also measured (wing chord for estimation of age in days, tarsus width and depth for estimation of sex, see 3, 11), and weighed (for nutritional status), sampled (feather and blood, in Sweden archived in the national Specimen Data Bank for chemical analyses and genetic studies), and ringed within an international colour ringing programme, for identifications in the field (8). Dead eggs and shell pieces are collected for measurements, investigation of contents and chemical analyses, for studies on relationships with reproduction (cf. Fig. 5). Also shed feathers from adults are collected at all sites and archived. These materials are used in the assessment of other parameters/indicators.
Contaminant burdens
Tissue and egg samples of white-tailed sea eagle have contained among the highest residue concentrations of persistent organochlorine contaminants and heavy metals in the Baltic and in the world (13, 15, 18, 19). Being highly exposed to persistent chemicals, predatory birds are useful in detecting the presence of ”new” pollutants that are potentially harmful, as illustrated by the discovery of PCB in 1966 in a Baltic white-tailed sea eagle (17) and the discovery of the flame retardant congener PBD-209 in peregrine falcon eggs in 2004 (20).
Chemical analyses of samples of contents from collected dead eggs provide possibilities to study relationships between the concentrations of contaminants and reproduction. In free-ranging birds this is usually done on a population level, but more detailed studies can also be made when individual breeding pairs can be followed over time periods. In addition to being highly exposed to persistent chemicals, the white-tailed sea eagle has features that are favourable from this monitoring perspective. Territorial adults on the Baltic Sea coast are mainly sedentary and thus reflect the regional contaminant situation. Mates of pairs are generally faithful to each other and to their breeding sites, and the sites are commonly used over many generations of eagles, providing good opportunities for long-term studies. A large portion of the breeders in the Baltic region is currently ringed, improving the possibilities for study of individual birds over time.
Studies of Baltic sea eagles have revealed strong correlations between residue concentrations of DDTs and PCBs and reproduction (6, 10, 12, 15). Studies of individual eagles over time has shown that females that were exposed to high concentrations of contaminants during the 1960s and 1970s remained unproductive also after residue concentrations in their eggs had declined, indicating persistent effects from a previous exposure (12). Trends in productivity and in residue concentrations of DDE and PCB are shown in Figure 5. Residue concentrations of DDE have now declined below an estimated critical threshold level for effects on reproduction. Residue concentrations of brominated flame retardants have been investigated in eagle egg samples from four regions in Sweden – the Baltic Proper, Bothnian Sea, and inland freshwaters in southern Sweden and Lapland (21). No significant difference was found between the samples from the Baltic coast. Concentrations in the Baltic samples were three and six times higher than in inland samples from southern Sweden and Lapland, respectively. Investigations on other contaminants are in progress, in search for an explanation for the smaller brood size in the Bothnian Sea.
Nutritional condition of nestlings
Body mass can be indicative of food stress and health and is usually easily obtained when handling nestlings. An age-dependant increment in body mass naturally takes place in growing nestlings, and comparison of weights between nestlings must therefore be based on specimens of the same age. Wing length is strongly correlated to age in sea eagle nestlings (3, 11) and can be used as a proxy for age. A sub-sample (all nestlings available from 1977 to 1982) illustrates a considerable difference in weight between nestlings from the Swedish Baltic coast and from a population in Swedish Lapland (Fig. 6). In this case the difference was a result of food shortage. Age-specific body mass data from nestlings can also be used to monitor trends in condition and health within a population. Since sea eagles are sexually dimorphic (females are usually bigger than males), body mass records should ideally be assigned to either sex and treated separately. Tarsus width is a good indicator of sex in sea eagle nestlings (11). But mixed samples may be useful as well in the monitoring of trends, or to study differences between areas as exemplified in Figure 6. When sexing can be attained a higher resolution in the studies can be achieved (11).
Death reasons of found specimens
Eagles found dead in nature belong to the state in all countries around the Baltic Sea except Germany. This provides good opportunities for investigations of death reasons. In Sweden, state game normally goes to the Swedish Museum of Natural History, for registration, measurements, examination and preparation for the museum collections. Before being opened, all white-tailed sea eagles are inspected macroscopically for body condition and signs of trauma, and x-rayed to assess the presence of lead shot, fractures etc. Distributions of death reasons of sea eagles from Germany, Finland and Sweden are presented in (16) . In Sweden, organ samples are saved in the museum’s Specimen Data Bank from all reasonably fresh specimens. Analyses of heavy metals in archived samples of sea eagle liver and kidney tissue have been performed recently. The results for lead contamination revealed no decrease in lead concentrations over the study period 1981-2004, and indicate that a minimum of 14 % of investigated specimens were lethally poisoned from ingestion of lead ammunition (9). The results for mercury are in progress.
Data
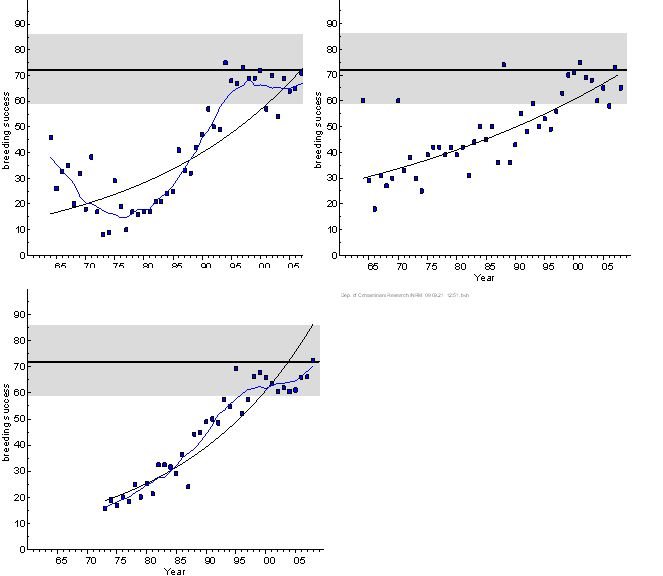
Figure 2. Breeding success (%) of white-tailed sea eagle on the Swedish coast of the Baltic Proper (upper left) and the Gulf of Bothnia (Bothnian Sea and Bothnian Bay), 1964–2008, and in Mecklenburg-Western Pomerania, Germany, 1973-2008 (lower). The blue line included in the set of breeding success data represents a LOESS smoother that explained significantly more than the linear regression line. Reference level with 95% confidence limits is given according to (7). Whether the reference level, estimated from data from the Swedish Baltic coast, is fully relevant for the German eagle population has not been validated.
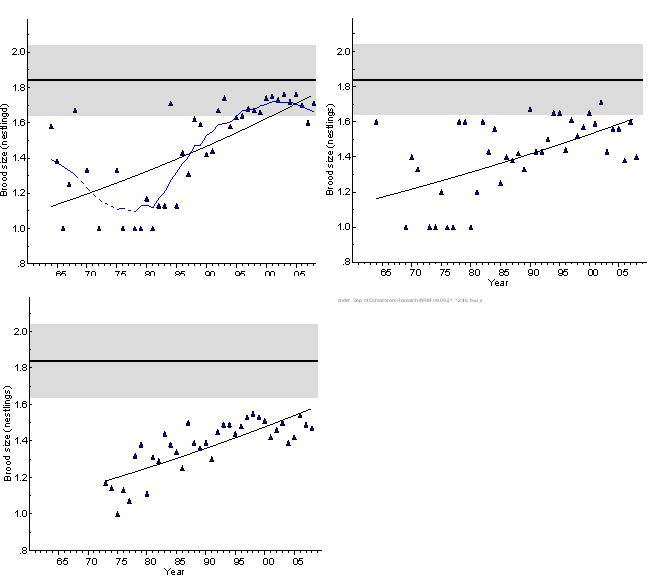
Figure 3. Mean nestling brood size of white-tailed sea eagle on the Swedish coast of the Baltic Proper (upper left) and the Gulf of Bothnia (Bothnian Sea and Bothnian Bay), 1964–2008, and in Mecklenburg-Western Pomerania, Germany, 1973-2008 (lower). The data set from Germany includes nests that were inspected only from the ground. Reference level with 95% confidence limits is given according to (7). Whether the reference level, estimated from data from the Swedish Baltic coast, is fully relevant for the German eagle population has not been validated.
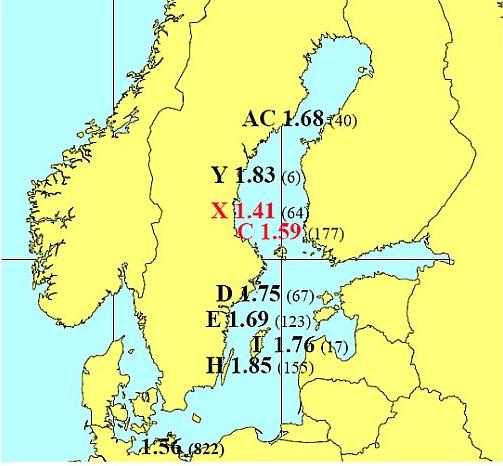
Figure 4. Mean nestling brood size 1996-2004 on the Swedish Baltic coastline,counties indicated by letters, and in Mecklenburg-Western Pomerania, (German data corrected for nests checked from the ground). Sample sizes given in brackets. The reference level up to 1950 based on data from the Swedish coast was 1.84, with 95 % confidence limits 1.64 - 2.04.
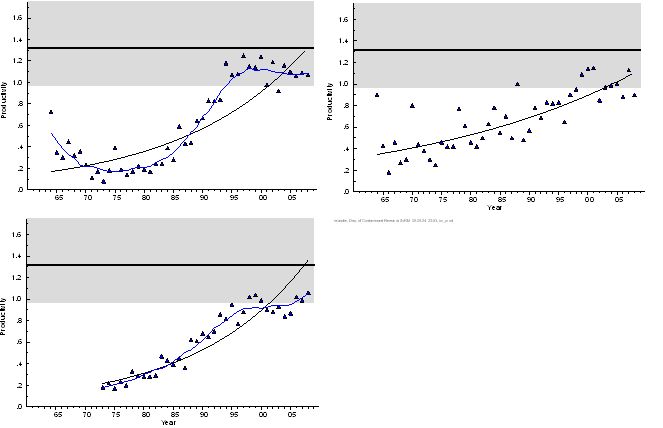
Figure 5. Mean annual productivity of white-tailed sea eagle on the Swedish coast of the Baltic Proper (upper left)and the Gulf of Bothnia (Bothnian Sea and Bothnian Bay), 1964–2008, and in Mecklenburg-Western Pomerania, Germany, 1973-2008 (lower). The data set from Germany includes nests that were inspected only from the ground. Reference level given with range based on confidence limits for breeding success and brood size according to (7). Whether the reference level, estimated from data from the Swedish Baltic coast, is fully relevant for the German eagle population has not been validated.
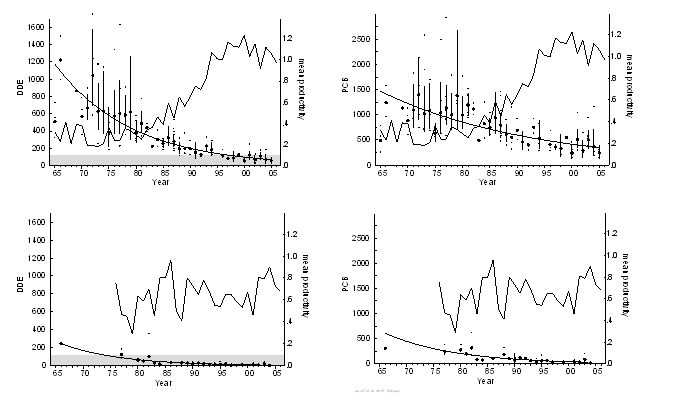
Figure 6. Mean annual productivity and residue concentrations of DDE and total-PCB in white-tailed sea eagle eggs, Swedish Baltic coast 1965-2005 (upper graphs) and a reference population in Swedish Lapland 1976-2005. Areas below a previously estimated LOEL for DDE (1) are shaded. Large dots = annual geometric means, small dots = individual clutches, vertical lines = 95 % confidence limits (for sample sizes > 3). Regression lines for DDE and PCB in the eggs decreased significantly during the study periods (p<0.001). Productivity of the coastal population increased significantly (p<0.001); there was no statistically significant change in productivity over the study period in the reference population in Lapland. From (10).
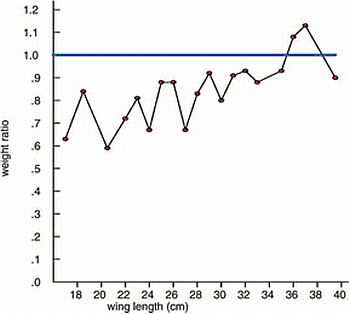
Figure 7. Weight ratio of nestlings from Lapland (n = 53) in relation to nestlings from the Baltic coast (n = 56, reference line 1.0) over ages 3.5–7 weeks as indicated by wing length in cm. From (10).
Metadata
Technical information
Data source: The National Swedish Monitoring Programme of Seas and Coastal areas/ National Environment Protection Agency; Swedish Museum of Natural History; Swedish Society for Nature Conservation (Project Sea Eagle).
German Data: Agency for Environment, Nature Conservation, and Geology of Mecklenburg-Western Pomerania
Description of data: Surveys of breeding populations and reproduction, sampling, sample preparation, storage in specimen bank and evaluation of results are carried out by the Department of Contaminant Research at the Swedish Museum of Natural History, Stockholm. Surveys of breeding populations and reproduction of reference freshwater populations are carried out by the Swedish Society for Nature Conservation (Project Sea Eagle), Stockholm. Chemical Analysis is carried out at the Institute of Applied Environmental Research at Stockholm University.
In Germany, Western Pomerania, data are collected by voluntary ornithologists, co-ordinated by the “Project group for large bird species” under the auspices of the Agency for Environment, Nature Conservation and Geology. The country-wide white-tailed sea eagle data are compiled by Peter Hauff , who submits the annual reports to the mentioned governmental agency.
Geographical coverage: see figures.
Temporal coverage: see figures.
Methodology and frequency of data collection: see (1-3).
Methodology of data processing: Simple log-linear regression analysis has been carried out to investigate average changes over time. To check for significant nonlinear trend components, a LOESS smoother was applied and an analysis of variance was used to check whether the smoother explained significantly more than the regression line. Statistical power analyses were used to estimate the minimum annual trend likely to be detected at a statistical power of 80% during a monitoring period of 10 years. To investigate the possible effect of a future reduced sampling scheme, repeated random sampling (5000 times) from 1991 to 2006 in the current database was carried out, simulating a maximum of 50, 25, 20, 15, and 10 records each year. Contingency analysis, using the G-test with Williams correction, a log-likelihood ratio test, was applied for comparisons between geographical regions and time periods. For references see (10).
Quality of information
Minimum detectable yearly trend (%) for a 10-year monitoring period at a statistical power of 80 % has been estimated for Swedish data for different sample sizes, based on random sampling from data collected during 1991 – 2006 (2). Minimum detectable trends based on the raw data set between 1991–2006 (with a varying annual n of observations) was 1.3 % for brood size (Baltic Proper), 2.0 % for breeding success (Gulf of Bothnia) and 3.0 % for productivity (Gulf of Bothnia).
References
1. Hauff, P. (2009): Zur Geschichte des Seeadlers Haliaeetus albicilla in Deutschland. Denisia 27: 7-18
2. Hauff, P. & L. Wölfel (2002). Seeadler (Haliaeetus albicilla) in Mecklenburg-Vorpommern im 20. Jahrhundert.
Corax, Special Issue 1, 15-22
3. Helander, B. (1981). Nestling measurements and weights from two white-tailed eagle populations in Sweden. Bird
Study 28, 235–241.
4. Helander, B. (1985). Reproduction of the white-tailed sea eagle Haliaeetus albicilla in Sweden. Holarct. Ecol.
8(3):211-227.
5. Helander, B. (1994a). Pre-1954 breeding success and productivity of white-tailed sea eagles Haliaeetus albicilla in
Sweden. In: Raptor Conservation Today. Meyburg, B.-U.& Chancellor, R.D. (eds). WWGBP/The Pica Press, pp. 731–733.
6. Helander, B. (1994b). Productivity in relation to residue levels of DDE in the eggs of white-tailed sea eagles
Haliaeetus albicilla in Sweden. . Pp. 735-738 in: Meyburg, B.-U. & Chancellor, R.D. (eds.), Raptor Conservation Today.WWGBP/The Pica Press
7. Helander, B. (2003a). The white-tailed Sea Eagle in Sweden—reproduction, numbers and trends. In: SEA EAGLE
2000. Helander, B., Marquiss, M. and Bowerman, B. (eds). Åtta.45 Tryckeri AB, Stockholm, pp. 57–66.
8. Helander, B. (2003b). The international colour ringing programme – adult survival, homing and the expansion of
the white-tailed sea eagle in Sweden. In: SEA EAGLE 2000. Helander, B., Marquiss, M. and Bowerman, B. (eds). Åtta.45 Tryckeri AB, Stockholm, pp. 145-154.
9. Helander, B., J. Axelsson, H. Borg, K. Holm.& A. Bignert (2009). Ingestion of lead from ammunition and lead
concentrations in white-tailed sea eagles (Haliaeetus albicilla) in Sweden. Sci. Tot. Environ. 407:5555–5563.
10. Helander, B., A. Bignert.& L. Asplund (2008). Using Raptors as Environmental Sentinels: Monitoring the White-
tailed Sea Eagle (Haliaeetus albicilla) in Sweden. Ambio 37(6):425-431.
11. Helander, B., F. Hailer & C. Vila, C. (2007). Morphological and genetic sex identification of white-tailed eagle
Haliaeetus albicilla nestlings. J. Ornithol. 148, 435–442.
12. Helander, B., A. Olsson, A. Bignert, L. Asplund & K. Litzén (2002). The role of DDE, PCB, coplanar PCB and
eggshell parameters for reproduction in the white-tailed sea eagle (Haliaeetus albicilla) in Sweden. Ambio 31(5):386-403.
13. Helander, B., M. Olsson & L. Reutergårdh (1982). Residue levels of organochlorine and mercury compounds in
unhatched eggs and the relationships to breeding success in white-tailed sea eagles Haliaeetus albicilla in Sweden. Holarct. Ecol. 5(4):349-366.
14. Helander, B. & T. Stjernberg (eds.) (2003). Action Plan for the conservation of White-tailed Eagle (Haliaeetus
albicilla). Recommendation 92/2002, adopted by the Standing Committee of the Bern Convention in Dec., 2002. BirdLife International. 51 pp.
15. Henriksson, K., E. Karppanen & M. Helminen (1966). High residue levels of mercury i Finnish white-tailed
eagles. Orn. Fenn. 43:38-45.
16. Herrmann, C., O. Krone, T. Stjernberg & B. Helander (2009). Population Development of Baltic Bird Species:
White-tailed Sea Eagle (Haliaeetus albicilla). HELCOM Indicator Fact Sheets 2009, in press Online., http://www.helcom.fi/environment2/ifs/en_GB/cover/
17. Jensen, S. 1966. Report on a new chemical hazard. New Scient. 32:612.
18. Jensen, S., A.G. Johnels, M. Olsson & T. Westermark (1972). The avifauna of Sweden as indicators of
environmental contamination with mercury and organochlorine hydrocarbons. Proc. Int. Orn. Congr. 15:455-465.
19. Koivusaari, J., I. Nuuja, R. Palokangas & M. Finnlund (1980). Relationships between productivity, eggshell
thickness and pollutant contents of addled eggs in the population of white-tailed eagles Haliaeetus albicilla L. in Finland during 1969–1978. Environ. Pollut. (Ser. A) 23:41–52.
20. Lindberg, P., U. Sellström, L. Häggberg & C.A. De Wit (2004). Higher brominated diphenyl ethers and
hexabromocyclododecane found in eggs of peregrine falcons (Falco peregrinus) breeding in Sweden. Environ. Sci. Technol. 38:93–96.
21. Nordlöf, U., B. Helander, A. Bignert & L. Asplund (2007). Polybrominated Flame retardants in eggs from Swedish White-Tailed Sea Eagle (Haliaeetus albicilla). Organohalogen Compounds 69:2728-2731.
For reference purposes, please cite this indicator fact sheet as follows:
[Author’s name(s)], [Year]. [Indicator Fact Sheet title]. HELCOM Indicator Fact Sheets 2009. Online. [Date Viewed], http://www.helcom.fi/environment2/ifs/en_GB/cover/.
Last updated: 28 September 2009