Impacts of invasive phytoplankton species on the Baltic Sea ecosystem in 1980-2008
|
||||
Key Message
![]() There are nine species of phytoplankton identified as non-native for the Baltic Sea, of them only one species, the dinoflagellate Prorocentrum minimum (Pavillard) Schiller, can be categorized as an invasive species, which is spreading and causing significant impacts on plankton community, habitat and ecosystem functioning.
There are nine species of phytoplankton identified as non-native for the Baltic Sea, of them only one species, the dinoflagellate Prorocentrum minimum (Pavillard) Schiller, can be categorized as an invasive species, which is spreading and causing significant impacts on plankton community, habitat and ecosystem functioning.
![]() During the recent three decades (1980-2008) the general trend was an increase in abundance and distribution range of P. minimum. The magnitude of impacts caused by this species varied in large extent, from 0 to 4, within the bioinvasion impact assessment scale (0 – no impact, 1 – weak, 2 – moderate, 3 – strong, 4 – massive).
During the recent three decades (1980-2008) the general trend was an increase in abundance and distribution range of P. minimum. The magnitude of impacts caused by this species varied in large extent, from 0 to 4, within the bioinvasion impact assessment scale (0 – no impact, 1 – weak, 2 – moderate, 3 – strong, 4 – massive).
Relevance of the indicator for describing developments in the environment
According to the EU Marine Strategy Framework Directive the Good Environmental Status should be defined in accordance with 11 qualitative descriptors, one of them being “Non-indigenous species”. The non-indigenous species (syn.: alien, non-native, exotic, introduced) are those which were introduced outside their natural range and dispersal potential. Their presence in the given region is due to intentional or unintentional introduction by humans (DAISIE, 2009). Some of these species are categorized as “Invasive alien species”: established alien species that reproduce in large number and are spreading over a large area, thus rapidly extending their range. Invasive alien species usually cause significant impacts on native communities, habitats, ecosystem functioning, and may harm economy and/or human health. Impacts of alien species may be interpreted as decline in ecological quality resulting from changes in biological, chemical and physical properties of aquatic ecosystems (Elliott, 2003). These changes include (but are not confined to): alteration of native communities; algal blooms; alteration of oxygen and nutrient content, pH and transparency of water.
Policy relevance and policy references
Invasive alien species are listed among the most important factors threatening the aquatic biodiversity in the Baltic Sea, together with eutrophication, contaminants, overfishing and destruction of habitats (EC Communication 2009). However the information on the impacts caused by the invasive alien species (bioinvasion impacts) is still scarce, especially for phytoplankton species.
Within two recent EU-funded projects (ALARM and DAISIE), a European team of researchers has developed a biopollution index in order to classify the impacts of invasive alien species on native species, communities, habitats and ecosystem functioning based upon their abundance and distribution range (ADR) (Olenin et al. 2007). Five classes of ADR were defined, ranking an alien species from low abundance in a few localities (A) to occurrence in high numbers in all localities (E). The authors have also considered three categories of impacts, whose magnitude is ranked on five levels ranging from no impact (0) through weak (1), moderate (2), strong (3), massive (4), namely:
Impact on native species and communities (from C0 to C4)
Impact on habitats (from H0 to H4)
Impact on ecosystem functioning (from E0 to E4).
Assessment
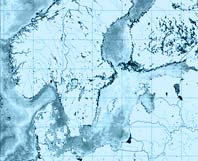
According to the Baltic Sea Alien Species Database (2009) there are nine non-indigenous phytoplankton species in the Baltic Sea, five diatoms: Coscinodiscus wailesii, Odontella sinensis, Pleurosigma simonsenii, Pleurosira leavis f. polymorpha and Thalassiosira punctigera and four dinophytes: Alexandrium tamarense, Gymnodinium catenatum, Karenia (Gyrodinium) mikimotoi and Prorocentrum minimum. Among these alien species only P. minimum may be classified as invasive species, because it was spreading during the assessment periods (1980-2008) and caused significant impacts. All other species occurred in low numbers (negligible in comparison to native phytoplankton) and did not spread to the east of Arkona Sea, therefore their impact on the biodiversity and ecosystem functioning of the Baltic Sea is considered as insignificant. The assessment is made for P. minimum for five years periods (1980–1984, 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2008) in eleven sub-regions of the Baltic Sea. During these years the species gradually penetrated to the Baltic Sea from Kattegat area (Figure 1). The abundance of P. minimum varied in large extent: from very small quantities (~100 cells L-1) or, sometimes, total absence in phytoplankton samples to massive development and presence in virtually all samples. The species was present mostly in summer – autumn phytoplankton.
Figure 1. Invasion of the dinoflagellate P. minimum into the Baltic Sea (Pedersen 1983; Witek & Plinski 2000; Hajdu et al 2000).
Impacts on the community was estimated according to the level of dominance of the P. minimum in total phytoplankton biomass, which varied from C0 (no impact) to C4 (massive impact). The later was recorded only once: in 1997 in Polish coastal waters (Gdynia harbor) during the outbreak of P. mimimum its abundance reached up to 350 million cells L-1 with relative biomass up to 98%. Situations where the invasive alien species dominated over native species in terms of biomass, yet former native dominant species were still present were determined as strong impact (C3); such situations were observed in 24% of case studies (Figure 2a). Impacts of P. mimimum on the pelagic habitat were more difficult to assess because they remain largely unstudied. Usually, the color of water has been changed to light brownish during the mass development of this species; also changes in pH and transparency of water were observed. Moreover, P. minimum seems to be a good competitor when nitrate (or other inorganic nutrient) is exhausted from the water in the early phase of the bloom and high concentrations of total nitrogen are available (Pertola et al 2005). The impacts on habitat were estimated in most cases at level H0 (no impact) to H2 (moderate impact) and as H3 (strong) in case of the species outbreak in Gdynia harbour (Figure 2b). Impacts of invasive species on ecosystem functioning may cause shifts in trophic nets and alteration of energy and organic material flow. So far there are no studies showing the role of P. minimum in alteration of the Baltic Sea food webs. However, because this species is known as mixotrophic (Stoecker et al 1997), it may be stated that at least shifts in dominant functional groups have occurred because in summer-autumn period usually autotrophic phytoplankton species (cyanobacteria, diatoms) have been dominant in communities. This effect should be most pronounced during the period of mass development of P. minimum, even if there were other (native) mixotrophic species in the system (Figure 2c).
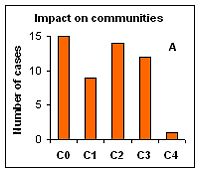
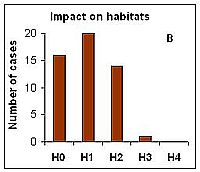
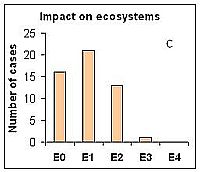
Figure 2. Assessed level of bioinvasion impact of the dinoflagellate P. minimum on native phytoplankton community (A), pelagic habitat (B) and ecosystem functioning (C) in 11 Baltic sub-regions during 5 years assessment periods from 1980 to 2008 (0 – no impact, 1 – weak, 2 – moderate, 3 – strong, 4 – massive).
Overall level of bioinvasion impacts (BPL – biopollution level index) of P. minimum varied greatly between different sub-regions in different assessment periods (Figure 3). This species successfully established itself in the entire Baltic Sea except the Gulf of Bothnia during the last two decades and become a summer-autumn bloom forming species, although it occurs irregularly between years. Even if the massive level of bioinvasion impact was recorded only in one case so far, this may repeat in the future in other areas of the Baltic Sea, because the situations of strong impacts have been often observed. During the first 5-years period (1980-1984) the species was in the stage of arrival to the Baltic Sea and establishment (according to the invasion phases scheme, Reise et al 2006). It occupied only south-western (Belt Sea, including Mecklenburg and Kiel Bights, and Arkona Sea) sub-region of the Baltic Sea, causing the highest bioinvasion impact at the level BPL=1. The expansion phase continued up to the 4th five years period; P. minimum reached the limit of its possible distribution in the Baltic Sea. During the expansion periods the species bioinvasion impact level varied greatly between sub-regions from BPL=0 to BPL=4. During the latest assessment periods 2000-2004 and 2005-2008 the species reached the adjustment phase (sensu Reise et al 2006) in all evaluated sub-regions.
Figure 3. Dynamics of the bioinvasion impacts caused by the dinoflagellate Prorocentrum minimum in the assessment units (Baltic sub-regions) during the five years assessment periods and the highest level of impact reached (“Weak” – green, “Moderate" – yellow, “Strong” – orange, “Massive” – red circle).
Technical information
1. Source: Estonian, Latvian, Lithuanian, Polish, German and Swedish national monitoring data.
2. Description of data: Abundance (cells L-l), relative biomass (%), distribution of alien species of phytoplankton (Prorocentrum minimum) at monitoring stations in eleven sub-regions of the Baltic Sea.
3. Geographical coverage: Entire Baltic Sea
4. Temporal coverage: 1980-2008.
5. Methodology and frequency of data collection: Information based on national monitoring samples. Data from the same sub-basin pooled. Phytoplankton samples analyzed and identified by phytoplankton experts, using the mandatory HELCOM methods (http://www.helcom.fi/groups/monas/CombineManual/AnnexesC/en_GB/annex6). The bioinvasion impact assessment was performed using the online Biopollution assessment system http://corpi.ku.lt/~biopollution/ which makes possible translation of existing data on invasive species impacts into uniform biopollution measurement units and accumulates data on bioinvasion impacts of organisms in different aquatic ecosystems. All data used in the present assessment is available at the above Internet site.
6. Methodology of data manipulation: The assessment is made for P. minimum for five years periods (1980–1984, 1985–1989, 1990–1994, 1995–1999, 2000–2004, 2005–2008) in eleven sub-regions of the Baltic Sea. A five year period in a particular sub-region was considered as a case study; in total there were 51 such case studies. The overall bioinvasion impact (Biopollution level, BPL) for the assessment unit is determined according to the greatest impact level for the alien species which was noticed during 1980-2008 evaluation period.
References
EC Communication 2009. Commission of The European Communities, Brussels, 10.6.2009 Sec(2009) 712 Commission Staff Working Document.
DAISIE, 2009. DAISIE. Handbook of Alien Species in Europe. Invading Nature - Springer Series In Invasion Ecology. V. 3, Springer: 399.
Elliott, M., 2003. Biological pollutants and biological pollution – an increasing cause for concern. Marine Pollution Bulletin 46, 275–280.
Hajdu, S., Edler, L., Olenina, I., Witek, B., 2000. Spreading and Establishment of the Potentially Toxic Dinoflagellate Prorocentrum minimum in the Baltic Sea. J Internat Rev Hydrobiol 85(5-6): 561–575.
Olenin, S., Minchin, D., Daunys, D., 2007. Assessment of biopollution in aquatic ecosystems, Mar. Pollut. Bull. doi:10.1016/j.marpolbul.2007.01.010.
Baltic Sea Alien Species Database, 2009. In: Olenin, S., Leppäkoski, E., Daunys, D. (Eds.), <http://www.ku.lt/nemo/alien_species_search.html> (accessed online 18.08.09).
Pedersen, S.M., 1983. Udbredelsen af Prorocentrum minimum i danske farvande i august 1983. Rapport til miljøstyrelsens havforureningslaboratorium. Marine Identification Agency ApS, Skodsborg. pp.7.
Pertola, S., Kuosa, H., Olsonen, R., 2005. Is the invasion of Prorocentrum minimum (Dinophyceae) related to the nitrogen enrichment of the Baltic Sea? Harmful Algae 4: 481–492.
Stoecker, D.K., Li, A., Coats, D.W., Gustafson, D.E., Nannen, M.K., 1997. Mixotrophy in the dinoflagellate Prorocentrum minimum. Mar Ecol Prog Ser 152: 1-12.
Reise, K., Olenin, S., Thieltges, D.W., 2006. Are aliens threatening European aquatic coastal ecosystems? Helgoland Marine Research 60(2), 106–112.
Witek, B., Plinski, M., 2000. The first recorded bloom of Prorocentrum minimum (PAVILLARD) SCHILLER in the coastal zone of the Gulf of Gdansk, Oceanologia 42: 29-36.
Footnotes
1 Unifob Environmental Research, Thormøhlensgt. 49, Bergen, Norway
2 Coastal research and Planning Institute, Klaipeda University, Manto str. 84, Klaipeda, Lithuania
3 Department of Systems Ecology, Stockholm University, SE-10691, Stockholm, Sweden
4 Leibniz-Institute for Baltic Sea Research, Seestr. 15, Warnemünde, Germany
5 Institute of Aquatic Ecology, Marine Monitoring Center, Daugavgrivas str. 8, Riga, Latvia
6 Sea Fisheries Institute, Kołłataja 1, PL 81-332 Gdynia, Poland
7 Estonian Marine Institute, University of Tartu, Mäealuse str. 10a, Tallinn, Estonia
For reference purposes, please cite this indicator fact sheet as follows:
[Author’s name(s)], [Year]. [Indicator Fact Sheet title]. HELCOM Indicator Fact Sheets 2009. Online. [Date Viewed], http://www.helcom.fi/environment2/ifs/en_GB/cover/.
Last updated: 7 September 2009