Population Development of Baltic Bird Species: White-tailed Sea Eagle (Haliaeetus albicilla)
|
||||
Key message
At the beginning of the 20th century, as a consequence of severe persecution the White-tailed Sea Eagle faced extinction all around the Baltic Sea. In Denmark, the species even disappeared after 1911, and the same happened in Lithuania and the Kaliningrad Region of Russia.
Due to protection measures, the population started to recover during the 1920s, but the positive trend was reversed from the mid-1950s to the early 1980s by the harmful effects of chemical pollutants (DDT, PCBs, and mercury) on mortality, fertility and reproduction success. Due to the ban of DDT and other pesticides in the early 1970s, the reproduction parameters started to improve at the beginning of the 1980s, and returned to normal levels in the mid-1990s (Helander et al. 2002). This resulted in an increase of breeding pair numbers in all Baltic Sea countries, which started during the 1980s and is still ongoing. From 1991 to 2007 the total Baltic population grew from about 680-690 bp to 2,100-2,250 bp.
Despite the positive population development the White-tailed Sea Eagle still requires concern of nature conservationists since anthropogenic factors are the main mortality reasons in many regions of the Baltic. Intoxications (especially lead intoxication from hunting ammunition) and collisions with trains, power lines and windpower rotors are the most significant mortality factors. Illegal persecution is also still existing, though of lower significance. It is likely that anthropogenic mortality has an impact on the population development, i.e. it is slowing down the current growth and expansion of the range. The White-tailed Sea Eagle as a top predator has been in the past, and will be in the future a sensitive indicator for the condition of the environment.
Results and assessments
Relevance of the indicator for describing developments in the environment
The White-tailed Sea Eagle is an example for species which have severely suffered by persecution and later by hazardous substances. Strict conservation measures and the ban of hazardous substances allowed it to recover. However, until today anthropogenic mortality factors (especially intoxication and collisions) exert a pressure on the population development. Since these factors are not only affecting the White-tailed Sea Eagle, but also other birds of prey, the species is a suitable indicator for the impact of certain human activities on bird species.
Policy relevance and policy references
The White-tailed Sea Eagle is protected by the provisions of the EU Bird Directive (79/409/EEC), which are implemented by the Member States into national law. This means, the legal protection status of the White-tailed Sea Eagle is similar in all Baltic Sea states except of Russia. The species is listed in Annex I of the Bird Directive, i.e., there is an obligation for the Member States to establish Special Protected Areas (SPA).
The White-tailed Sea Eagle is a “flagship species” for nature conservation. It is an example that strict conservation measures and firm international actions against environmental contamination with hazardous substances may allow species to recover after having been close to extinction.
Since anthropogenic mortality factors still exerting high pressure on the species, the White-tailed Sea Eagle is still an important indicator for human impacts on bird species, especially birds of prey.
Provisional conservation targets
Population development and size should not be significantly affected by anthropogenic factors. Anthropogenic mortality reasons should be less than 50 % of the total mortality.
Assessment
White-tailed Sea Eagle Population Development in the Baltic Sea Area
The White-tailed Sea Eagle colonises coastal and inland lake areas of all Baltic countries. These countries, together with Norway, host the major part of the European population, which nowadays probably accounts for more than 50 % of the global population.
Though the White-tailed Sea Eagle is not a “true” coastal species, it reaches remarkably high concentrations in coastal areas. The Odra lagoon area, for instance, has been known as one of the last density centres during the first half of the 20th century (Mizera 2002), and today still shows the highest density of breeding pairs in Central Europe (Hauff et al. 2007). In Lithuania, a concentration of breeding sites in the Nemunas delta and around the Curonian Lagoon is remarkable (Dementavičius 2007). In Finland, about 80-90 % of the breeding pairs are found in coastal regions (Figure 3). In Sweden, the proportion of the coastal breeding pairs currently amounts about 65 % (Figure 2). The re-colonisation of inland Sweden starting from the 1980s was made up of birds from the Baltic coast, as population density increased there (Helander 2003).
At the beginning of the 20th century, as a consequence of severe persecution the White-tailed Sea Eagle was close to extinction all around the Baltic Sea. In Denmark, the species even disappeared after 1911; in Mecklenburg-Western Pomerania, in 1913 only 23 breeding pairs (bp) were known, and the Polish population of that time is estimated at about 30 bp (Hauff & Wölfel 2002; Kollmann et al. 2002). In Lithuania and the Kaliningrad Region of Russia the White-tailed Sea Eagle disappeared for a long time.
Due to protection measures, the population started to recover during the 1920s, but the positive trend was reversed from the mid-1950s to the early 1980s by the harmful effects of pesticides and other chemical pollutants (DDT, PCBs, and mercury) on fertility and reproduction success. The proportion of successful breeding pairs dropped down to only 20-30 %, and the reproduction success to 0.2-0.4 fledglings per breeding pair. As a consequence, the population remained stagnant or even decreased. The use of mercury as seed dressing and paper mill industries imposed another threat, resulting in lethal mercury concentrations in White-tailed Sea Eagles between 1967 and 1978 (Oehme 1981). Owing to the ban of DDT and other pesticides in the early 1970s, the reproduction parameters started to improve at the beginning of the 1980s, and returned to almost normal levels in the mid-1990s (Helander & Bignert 2008; HELCOM 2009). The improved reproduction of the White-tailed Sea Eagle resulted in a still ongoing increase of the breeding population all around the Baltic, showing very similar trends in the south-western, central and north-eastern parts of the sea (Figure 1-3; Table 1). The total Baltic population grew from about 680-690 bp in 1991 to 1,150-1,200 bp in 1998, and 2,100-2,250 bp in 2007, i.e. it grew by more than 200 % within 16 years (Table 1).
Simultaneously, the species returned to territories abandoned in the past (e.g. Lithuania 1987, Denmark 1995). In recent years, a range expansion to the west (western and south-western parts of Germany) has been observed, and in 2006 the first breeding pair was recorded in the Netherlands (Roder & Bijlsma 2006).
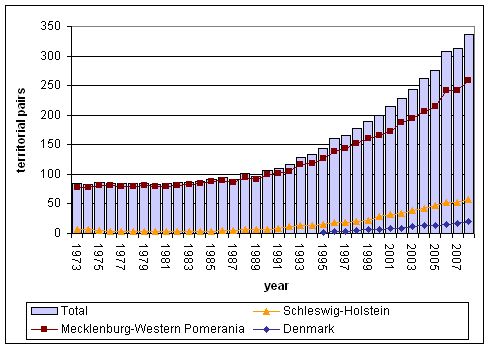
Figure 1: The population development of the White-tailed Sea Eagle in the south-western Baltic (Denmark, Schleswig-Holstein, and Mecklenburg-Western Pomerania) 1973-2008. Data from the Project Group for Conservation of Large Birds Mecklenburg-Western-Pomerania, Project Group for White-tailed Eagle Conservation Schleswig-Holstein e.V. & Dansk Ornitologisk Forening.
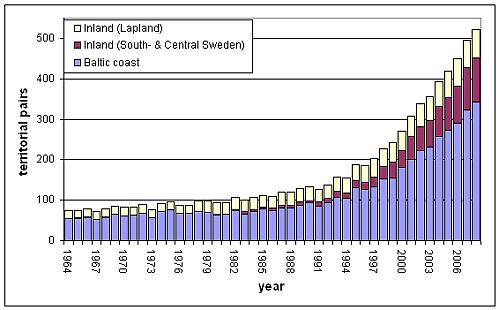
Figure 2: Population development of the White-tailed Sea Eagle in Sweden 1964-2008. Data from the ‘Project Sea Eagle’/Sweden, Swedish Society for Nature Conservation and Swedish Museum of Natural History.
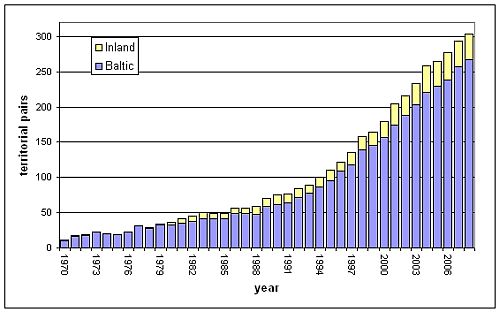
Figure 3: Population development of the White-tailed Sea Eagle in Finland 1970-2008. About 85-90 % of the breeding pairs are found in coastal areas. The localisation “inland” mainly refers to breeding sites in Lapland. Data from WWF Finland's White-tailed Sea Eagle working group (Stjernberg et al. 2009).
Table 1: Development of the population of White-tailed Sea Eagle in the Baltic Sea riparian states (HELCOM 2009).
| Country | Territorial pairs | Current | |||
| 1991 | 1998 | 2007 | |||
| Denmark | 0 | 5 | 17 | ++ | |
| Estonia | 40 | 60 | 150-170 | ++ | |
| Finland | 77 | 158 | 294 | ++ | |
| Germany - SH | 8 | 20 | 53 | ++ | |
| Germany - MV | 102 | 153 | 242 | ++ | |
| Latvia | 5-8 | 11 | 25 | ++ | |
| Lithuania | 7 | 25-30 | 90 | ++ | |
| Poland | 300 | 500 | 700-800 | ++ | |
| Russia, Kaliningrad region | 1-4 | 5-6 | >20 | ++ | |
| Russia, St. Petersburg region | 15 | 20 | 25-30 | + | |
| Sweden | 127 | 227 | 496 | ++ | |
| Baltic Sea riparian states | 680-690 | 1 150-1 200 | 2 100-2 250 | ++ | |
Mortality factors and risk analysis
The positive population development of the White-tailed Sea Eagle in recent years should not give reason to deny negative impacts caused by humans. Anthropogenic mortality factors are still of significant importance for the species and its population development. In Mecklenburg-Western Pomerania, from 1999-2008 a total of 236 White–tailed Sea Eagles found dead have been examined, and 70 % of the casualties could be attributed to anthropogenic factors (Figure 4). Considering that a certain proportion of birds which have died have not been found, because ill birds hide at places with a low risk of human disturbances, the importance of chronic intoxication could even be higher. Swedish and Finnish data suggest a similar magnitude of anthropogenic mortality factors (73 and 72 %, respectively). However, it should always be considered that the probability of detecting carcasses is biased towards places with high human activity (streets, railways, wind power plants, power lines, etc.) unless the carcasses are not removed on the quiet to conceal the true cause of death. The likelihood of finding dead eagles also varies depending on the population density of humans; i.e. in lowly populated regions of Sweden and Finland the bias towards areas with high human activities is even higher than in highly populated countries like Germany.
The main anthropogenic mortality factors are intoxications, collisions, and electrocution. Among the first group, lead intoxication requires special attention, since it is currently one of the most important causes of death in White-tailed Sea Eagles, accounting for 28.4 % of the casualties in Germany (Figure 5) and 13.5 % in Sweden (Figure 6; Helander et al. 2009). Also in Finland, lead intoxication seems to be of high significance (Krone et al. 2006). Lead is ingested by the Eagles when feeding on dead animals which have been shot by hunters, but not removed (injured animals which have died later and not been found; unusable animals such as foxes, martens or crows), or when they prey on animals injured by hunting ammunition (e.g., waterfowl injured by buckshot). Furthermore, many hunters leave guts and other unusable remnants from shot game animals, also called gut piles, back at the hunting site or even use it for places to lure carnivores. These remnants contain lead fragments from the bullet, which are ingested by the eagles. The low pH value in the gizzard, the aqueous gastric juice containing enzymes, the long retention time and other physiological factors such as motility of the gizzard muscles and the ingesta including even bones are key factors for the dissolution of the metallic lead particles. Birds are sensitive to lead poisoning as proven in numerous studies. An actual overview on lead exposure and intoxications in birds from Europe has been provided by Mateo (2009). Feeding experiments have shown that very small amounts of lead are sufficient to kill eagles of the genus Haliaeetus (Pattee et al. 1981).
Other reasons for intoxications recorded in Germany are ingestion of poisoned baits (e.g. Carbofuran). Such baits are probably in most cases not meant for the eagles, and the practice in general is strictly prohibited by law. Nevertheless, several casualties of White-tailed Sea Eagles had to be attributed to this factor. Another threat factor is illegal persecution by shooting. In the German sample of analysed dead eagles (n=390; entire sample of White-tailed Sea Eagles found dead in Germany between 1996 and 2007 and analysed at the Leibniz Institute for Zoo and Wildlife Research, Berlin), three birds were killed by shooting (2x gun shot pellets, 1x rifle projectile), and another 13 birds harboured lead shot pellets in their bodies (Krone et al. 2009). From Denmark, there is a report of a bird shot in early spring 2009 (T. Bregnballe, pers. comm.). These data show that illegal predatory bird persecution is still existing.
Collisions account for at least 22 % of the casualties in the German sample, and 50 % in the Swedish sample. However, the real number is likely much higher, since both samples include traumata with unknown reasons (9 % in the German and 4 % in the Swedish study, respectively), of which some probably have been caused by collisions. Among the collisions those with trains are of special significance (15 % of the German and 33 % of the Swedish sample, respectively). White-tailed Sea Eagles are attracted by carcasses of animals killed by the trains, and while feeding on them they may become collision victims themselves. Collisions with power lines and, in recent times increasingly, windpower rotors are other anthropogenic mortality factors.
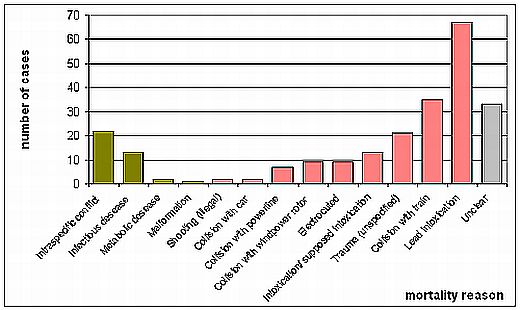
Figure 4: Mortality reasons of White-tailed Sea Eagles found dead in Mecklenburg-Western Pomerania 1999-2008; n = 236. Data from the Leibniz Institute for Zoo and Wildlife Research Berlin.
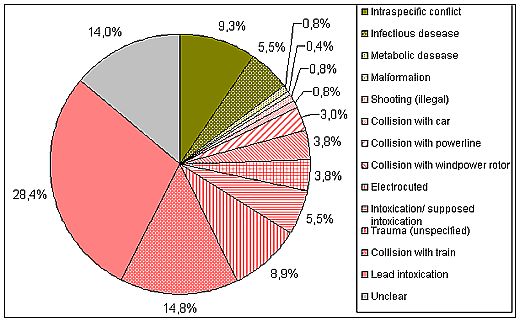
Figure 5: Percentage of mortality reasons of White-tailed Sea Eagles found dead in Mecklenburg-Western Pomerania 1999-2008; n = 236. About 81 % of the mortality[1] is caused by anthropogenic factors, lead intoxication being the dominant one. Data from the Leibniz Institute for Zoo and Wildlife Research Berlin.
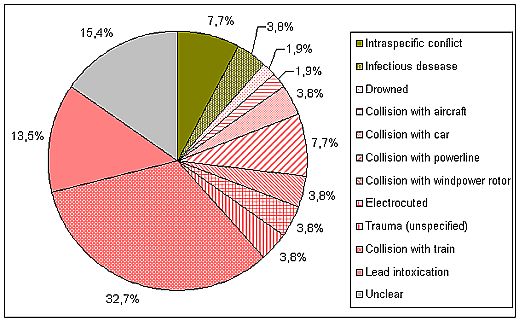
Figure 6: Percentage of mortality reasons of White-tailed Sea Eagles found dead in Sweden 2002-2007; n = 52. About 86 % of the mortality[2] is caused by anthropogenic factors, collision with trains being the dominant one, followed by lead intoxication. Results are preliminary waiting for complementary analyses. Data according to Helander & Bignert (2008).
The analysis of mortality factors from White-tailed Sea Eagles from Finland (Krone et al. 2006; n=11) revealed electrocution (n=4) and lead poisoning (n=2) as the most important causes of death. Unspecified trauma, drowning in fishing gear, and territorial fights are other mortality reasons (n=1 each). For one individual of the sample the mortality reason could not be identified. Again the majority (72 %) of the mortality factors are of anthropogenic origin. In one of the two lead intoxicated birds metallic lead particles from a bullet were detected. Collisions with road vehicles or trains are unlikely to occur, because there are almost no railways in the breeding areas of the species in SW and W Finland.
Metadata
Technical information
Data source: Annual surveys of the breeding population are organized and data are collected by the following institutions:
Denmark: National Environmental Research Institute (NERI), Aarhus University (published on the web site: http://www.dmu.dk)
Germany: Projektgruppe Seeadlerschutz Schleswig-Holstein e.V.; Agency for Environment, Nature Conservation, and Geology Mecklenburg-Western Pomerania
Finland: WWF Finland's White-tailed Sea Eagle working group
Sweden: ‚Project Sea Eagle’, Swedish Society for Nature Conservation and Swedish Museum of Natural History
Other data according to cited publications.
Geographical coverage: entire Baltic Sea area
Temporal coverage: see figures and table
Methodology and frequency of data collection: annual population surveys in Denmark, Schleswig-Holstein, Mecklenburg-Western Pomerania, Sweden and Finland;
In Sweden, monitoring of the White-tailed Sea Eagle is part of the national Swedish Environment Monitoring Programme.
Approximate population estimation in Sweden, Poland, Lithuania, Latvia, Estonia and Kaliningrad and St. Petersburg regions of Russia.
Quality of information: The reliability and quality of data is high.
References
Dementavičius, D. (2007): White-tailed Eagle (Haliaeetus albicilla) in Lithuania: Population numbers and trends 1900-2007. Acta Zoologica Lituanica 17, 281-285
Finnish Environment Institute SYKE (2008): The triumphant return of the White-tailed Eagle. http://www.environment.fi/download.asp?contentid=79476&lan=EN
Hauff, P. & L. Wölfel (2002): Seeadler (Haliaeetus albicilla) in Mecklenburg-Vorpommern im 20. Jahrhundert. Corax 19, Special Issue 1, 15-22
Hauff, P. & T. Mizera (2006): Verbreitung und Dichte des Seeadlers Haliaeetus albicilla in Deutschland und Polen: eine aktuelle Atlas-Karte. Vogelwarte 44, 134-136
Hauff, P., T. Mizera, J. Chavko, S. Danko, E. Ehmsen, K. Hudec, R. Probst & F. Vera (2007): Verbreitung und Dichte des Seeadlers Haliaeetus albicilla in sieben Ländern Mitteleuropas. Vogelwarte 45, 376-377.
Helander, B. (2003). The international colour ringing programme – adult survival, homing and the expansion of the white-tailed sea eagle in Sweden. In: Helander, B., Marquiss, M. and Bowerman, B. (Eds). SEA EAGLE 2000. Åtta.45 Tryckeri AB, Stockholm, pp. 145-154.
Helander, B., J. Axelsson, H. Borg, K. Holm & A. Bignert (2009). Ingestion of lead from ammunition and lead concentrations in white-tailed sea eagles (Haliaeetus albicilla) in Sweden. Sci. Tot. Environ. 407: 5555–5563.
Helander, B. & A. Bignert (2008): Predatory bird health – White-tailed Sea Eagle. HELCOM Indicator Fact Sheet 2008; viewed online 02/16/2009 http://www.helcom.fi/environment2/ifs/ifs2008/en_GB/predatory_bird/.
Helander, B., A. Olsson, A. Bignert, L. Asplund & K. Litzén (2002). The role of DDE, PCB, coplanar PCB and eggshell parameters for reproduction in the white-tailed sea eagle (Haliaeetus albicilla) in Sweden. Ambio 31(5):386-403.
HELCOM (2009): White-tailed eagle (Haliaeetus albicilla). In: Thematic Assessment on biodiversity and nature conservation. HELCOM Environmental Proceedings 116B, 77-78.
Kollmann, R., T. Neumann & B. Struwe-Juhl (2002): Bestand und Schutz des Seeadlers (Haliaeetus albicilla) in Deutschland und seinen Nachbarländern. Corax 19, Special Issue 1, 1-14
Krone O., T. Stjernberg, N. Kenntner, F. Tataruch, J. Koivusaari & I. Nuuja (2006): Mortality, helminth burden and contaminant residues in White-tailed Sea Eagles from Finland. Ambio 35: 98-104.
Krone, O., N. Kenntner & F. Tataruch (2009): Gefährdungsursachen des Seeadlers (Haliaeetus albicilla L. 1758). Denisia 27: 139-146.
Mebs, T. & D. Schmidt (2006): Die Greifvögel Europas, Nordafrikas und Vorderasiens. Kosmos-Naturführer.
Nyegaard, T. & M.B. Grell (2007): Truede og sjældne ynglefugle i Danmark 2007. DOF Report 10
Mateo, R. (2009): Lead poisoning in wild birds in Europe and the regulations adopted by different countries. In: R. T. Watson, M. Fuller, M. Pokras, and W. G. Hunt (Eds.). Ingestion of Lead from Spent Ammunition: Implications for Wildlife and Humans. The Peregrine Fund, Boise, Idaho, USA. DOI 10.4080/ilsa.2009.0107
Mizera, T. (2002): Bestandsentwicklung und Schutz des Seeadlers (Haliaeetus albicilla) in Polen im Verlauf des 20. Jahrhunderts. Corax 19, Special Issue 1, 85-91
Oehme, G. (1981): Zur Quecksilberrückstandsbelastung tot aufgefundener Seeadler, Haliaeetus albicilla, in den Jahren 1967–1978. Hercynia N. F. 18: 353-364.
Pattee, O.H., S.N. Wiemeyer, B.M. Mulhern, L. Sileo & J.W. Carpenter (1981): Experimental lead-shot poisoning in bald eagles. J. Wildl. Management 45: 806-810.
Roder F.E. de & Bijlsma R.G. (2006). Eerste broedgeval van de Zeearend Haliaeetus albicilla in Nederland. De Takkeling 14: 209-231.
Stjernberg, T., J. Koivusaari, J. Högmander, T. Ollila, S. Keränen, G. Munsterhjelm & H. Ekblom (2009): Suomen merikotkat 2007–2008. (Summary: Population size and nesting success of the White-tailed Sea Eagle (Haliaeetus albicilla) in Finland, 2007–2008). Linnut-vuosikirja 2008: 14–21.
Struwe-Juhl, B. (2002): Altersstruktur und Reproduktion des Seeadlerbrutbestandes (Haliaeetus albicilla) in Schleswig-Holstein. Corax 19, Special Issue 1, 51-62
Footnotes
[1] For the calculation of this percentage, the unclear cases have been excluded from the sample
[2] For the calculation of this percentage, the unclear cases have been excluded from the sample
For reference purposes, please cite this indicator fact sheet as follows:
[Author’s name(s)], [Year]. [Indicator Fact Sheet title]. HELCOM Indicator Fact Sheets 2009. Online. [Date Viewed], http://www.helcom.fi/environment2/ifs/en_GB/cover/.
Last updated: 11 December 2009